1 PANOBINOSTAT
2. BELINOSTAT
3' VORINOSTAT
4 MOCETINOSTAT
5 EZATIOSTAT
6 DACINOSTAT
7 GIVINOSTAT
8 TOPIROXOSTAT
9
10
WILL BE UPDATED SOON FEBUXOSTAT,
Tazemetostat
1 PANOBINOSTAT
Panobinostat
HDAC inhibitors, orphan drug
cas 404950-80-7
2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide
N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide (alternatively, N-hydroxy-3-(4-{[2-(2-methyl-1H-indol-3-yl)-ethylamino]-methyl}-phenyl)-acrylamide)
Molecular Formula: C21H23N3O2 Molecular Weight: 349.42622
- Faridak
- LBH 589
- LBH589
- Panobinostat
- UNII-9647FM7Y3Z
A hydroxamic acid analog histone deacetylase inhibitor from Novartis.
NOVARTIS, innovator
Histone deacetylase inhibitors
Is currently being examined in cutaneous T-cell lymphoma, CML and breast cancer.
clinical trials click here phase 3
DRUG SUBSTANCE–LACTATE AS IN http://www.google.com/patents/US7989639 SEE EG 31
Panobinostat (LBH-589) is an experimental drug developed by Novartis for the treatment of various cancers. It is a hydroxamic acid[1] and acts as a non-selective histone deacetylase inhibitor (HDAC inhibitor).[2]
Panobinostat is a cinnamic hydroxamic acid analogue with potential antineoplastic activity. Panobinostat selectively inhibits histone deacetylase (HDAC), inducing hyperacetylation of core histone proteins, which may result in modulation of cell cycle protein expression, cell cycle arrest in the G2/M phase and apoptosis. In addition, this agent appears to modulate the expression of angiogenesis-related genes, such as hypoxia-inducible factor-1alpha (HIF-1a) and vascular endothelial growth factor (VEGF), thus impairing endothelial cell chemotaxis and invasion. HDAC is an enzyme that deacetylates chromatin histone proteins. Check for
As of August 2012, it is being tested against Hodgkin’s Lymphoma, cutaneous T cell lymphoma (CTCL)[3] and other types of malignant disease in Phase III clinical trials, against myelodysplastic syndromes, breast cancer and prostate cancer in Phase II trials, and against chronic myelomonocytic leukemia (CMML) in a Phase I trial.[4][5]
Panobinostat is a histone deacetylase (HDAC) inhibitor which was filed for approval in the U.S. in 2010 for the oral treatment of relapsed/refractory classical Hodgkin’s lymphoma in adult patients. The company is conducting phase II/III clinical trials for the oral treatment of multiple myeloma, chronic myeloid leukemia and myelodysplasia. Phase II trials are also in progress for the treatment of primary myelofibrosis, post-polycythemia Vera, post-essential thrombocytopenia, Waldenstrom’s macroglobulinemia, recurrent glioblastoma (GBM) and for the treatment of pancreatic cancer progressing on gemcitabine therapy. Additional trials are under way for the treatment of hematological neoplasms, prostate cancer, colorectal cancer, renal cell carcinoma, non-small cell lung cancer (NSCLC), malignant mesothelioma, acute lymphoblastic leukemia, acute myeloid leukemia, head and neck cancer and gastrointestinal neuroendocrine tumors. Early clinical studies are also ongoing for the treatment of HER2 positive metastatic breast cancer. Additionally, phase II clinical trials are ongoing at Novartis as well as Neurological Surgery for the treatment of recurrent malignant gliomas as are phase I/II initiated for the treatment of acute graft versus host disease. The National Cancer Institute had been conducting early clinical trials for the treatment of metastatic hepatocellular carcinoma; however, these trials were terminated due to observed dose-limiting toxicity. In 2009, Novartis terminated its program to develop panobinostat for the treatment of cutaneous T-cell lymphoma. A program for the treatment of small cell lung cancer was terminated in 2012. Phase I clinical trials are ongoing for the treatment of metastatic and/or malignant melanoma and for the treatment of sickle cell anemia. The University of Virginia is conducting phase I clinical trials for the treatment of newly diagnosed and recurrent chordoma in combination with imatinib. Novartis is evaluating panobinostat for its potential to re-activate HIV transcription in latently infected CD4+ T-cells among HIV-infected patients on stable antiretroviral therapy.
Mechanistic evaluations revealed that panobinostat-mediated tumor suppression involved blocking cell-cycle progression and gene transcription induced by the interleukin IL-2 promoter, accompanied by an upregulation of p21, p53 and p57, and subsequent cell death resulted from the stimulation of caspase-dependent and -independent apoptotic pathways and an increase in the mitochondrial outer membrane permeability. In 2007, the compound received orphan drug designation in the U.S. for the treatment of cutaneous T-cell lymphoma and in 2009 and 2010, orphan drug designation was received in the U.S. and the E.U., respectively, for the treatment of Hodgkin’s lymphoma. This designation was also assigned in 2012 in the U.S. and the E.U. for the treatment of multiple myeloma.
Cardiovascular disease is the leading cause of morbidity and mortality in the western world and during the last decades it has also become a rapidly increasing problem in developing countries. An estimated 80 million American adults (one in three) have one or more expressions of cardiovascular disease (CVD) such as hypertension, coronary heart disease, heart failure, or stroke. Mortality data show that CVD was the underlying cause of death in 35% of all deaths in 2005 in the United States, with the majority related to myocardial infarction, stroke, or complications thereof. The vast majority of patients suffering acute cardiovascular events have prior exposure to at least one major risk factor such as cigarette smoking, abnormal blood lipid levels, hypertension, diabetes, abdominal obesity, and low-grade inflammation.
Pathophysiologically, the major events of myocardial infarction and ischemic stroke are caused by a sudden arrest of nutritive blood supply due to a blood clot formation within the lumen of the arterial blood vessel. In most cases, formation of the thrombus is precipitated by rupture of a vulnerable atherosclerotic plaque, which exposes chemical agents that activate platelets and the plasma coagulation system. The activated platelets form a platelet plug that is armed by coagulation-generated fibrin to form a biood clot that expands within the vessel lumen until it obstructs or blocks blood flow, which results in hypoxic tissue damage (so-called infarction). Thus, thrombotic cardiovascular events occur as a result of two distinct processes, i.e. a slowly progressing long-term vascular atherosclerosis of the vessel wall, on the one hand, and a sudden acute clot formation that rapidly causes flow arrest, on the other. This invention solely relates to the latter process.
Recently, inflammation has been recognized as an important risk factor for thrombotic events. Vascular inflammation is a characteristic feature of the atherosclerotic vessel wall, and inflammatory activity is a strong determinant of the susceptibility of the atherosclerotic plaque to rupture and initiate intravascular clotting. Also, autoimmune conditions with systemic inflammation, such as rheumatoid arthritis, systemic lupus erythematosus and different forms of vasculitides, markedly increase the risk of myocardial infarction and stroke.
Traditional approaches to prevent and treat cardiovascular events are either targeted 1) to slow down the progression of the underlying atherosclerotic process, 2) to prevent clot formation in case of a plaque rupture, or 3) to direct removal of an acute thrombotic flow obstruction. In brief, antiatherosclerotic treatment aims at modulating the impact of general risk factors and includes dietary recommendations, weight loss, physical exercise, smoking cessation, cholesterol- and blood pressure treatment etc. Prevention of clot formation mainly relies on the use of antiplatelet drugs that inhibit platelet activation and/or aggregation, but also in some cases includes thromboembolic prevention with oral anticoagulants such as warfarin. Post-hoc treatment of acute atherothrombotic events requires either direct pharmacological lysis of the clot by thrombolytic agents such as recombinant tissue-type plasminogen activator or percutaneous mechanical dilation of the obstructed vessel.
Despite the fact that multiple-target antiatherosclerotic therapy and clot prevention by antiplatelet agents have lowered the incidence of myocardial infarction and ischemic stroke, such events still remain a major population health problem. This shows that in patients with cardiovascular risk factors these prophylactic measures are insufficient to completely prevent the occurrence of atherothrombotic events.
Likewise, thrombotic conditions on the venous side of the circulation, as well as embolic complications thereof such as pulmonary embolism, still cause substantial morbidity and mortality. Venous thrombosis has a different clinical presentation and the relative importance of platelet activation versus plasma coagulation are somewhat different with an preponderance for the latter in venous thrombosis, However, despite these differences, the major underlying mechanisms that cause thrombotic vessel occlusions are similar to those operating on the arterial circulation. Although unrelated to atherosclerosis as such, the risk of venous thrombosis is related to general cardiovascular risk factors such as inflammation and metabolic aberrations.
Panobinostat can be synthesized as follows: Reduction of 2-methylindole-3-glyoxylamide (I) with LiAlH4 affords 2-methyltryptamine (II). 4-Formylcinnamic acid (III) is esterified with methanolic HCl, and the resulting aldehyde ester (IV) is reductively aminated with 2-methyltryptamine (II) in the presence of NaBH3CN (1) or NaBH4 (2) to give (V). The title hydroxamic acid is then obtained by treatment of ester (V) with aqueous hydroxylamine under basic conditions.
Panobinostat is currently being used in a Phase I/II clinical trial that aims at curing AIDS in patients on highly active antiretroviral therapy (HAART). In this technique panobinostat is used to drive the HI virus’s DNA out of the patient’s DNA, in the expectation that the patient’s immune system in combination with HAART will destroy it.[6][7]
 panobinostat
panobinostat
Panobinostat has been found to synergistically act with sirolimus to kill pancreatic cancer cells in the laboratory in a Mayo Clinic study. In the study, investigators found that this combination destroyed up to 65 percent of cultured pancreatic tumor cells. The finding is significant because the three cell lines studied were all resistant to the effects of chemotherapy – as are many pancreatic tumors.[8]
Panobinostat has also been found to significantly increase in vitro the survival of motor neuron (SMN) protein levels in cells of patients suffering fromspinal muscular atrophy.[9]
Panobinostat was able to selectively target triple negative breast cancer (TNBC) cells by inducing hyperacetylation and cell cycle arrest at the G2-M DNA damage checkpoint; partially reversing the morphological changes characteristic of breast cancer cells.[10]
Panobinostat, along with other HDAC inhibitors, is also being studied for potential to induce virus HIV-1 expression in latently infected cells and disrupt latency. These resting cells are not recognized by the immune system as harboring the virus and do not respond to antiretroviral drugs.[11]
Panobinostat inhibits multiple histone deacetylase enzymes, a mechanism leading to apoptosis of malignant cells via multiple pathways.[1]
The compound N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide (alternatively, N-hydroxy-3-(4-{[2-(2-methyl-1H-indol-3-yl)-ethylamino]-methyl}-phenyl)-acrylamide) has the formula
as described in WO 02/22577. Valuable pharmacological properties are attributed to this compound; thus, it can be used, for example, as a histone deacetylase inhibitor useful in therapy for diseases which respond to inhibition of histone deacetylase activity. WO 02/22577 does not disclose any specific salts or salt hydrates or solvates of N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide.
The compounds described above are often used in the form of a pharmaceutically acceptable salt. Pharmaceutically acceptable salts include, when appropriate, pharmaceutically acceptable base addition salts and acid addition salts, for example, metal salts, such as alkali and alkaline earth metal salts, ammonium salts, organic amine addition salts, and amino acid addition salts, and sulfonate salts. Acid addition salts include inorganic acid addition salts such as hydrochloride, sulfate and phosphate, and organic acid addition salts such as alkyl sulfonate, arylsulfonate, acetate, maleate, fumarate, tartrate, citrate and lactate. Examples of metal salts are alkali metal salts, such as lithium salt, sodium salt and potassium salt, alkaline earth metal salts such as magnesium salt and calcium salt, aluminum salt, and zinc salt. Examples of ammonium salts are ammonium salt and tetramethylammonium salt. Examples of organic amine addition salts are salts with morpholine and piperidine. Examples of amino acid addition salts are salts with glycine, phenylalanine, glutamic acid and lysine. Sulfonate salts include mesylate, tosylate and benzene sulfonic acid salts.
……………………………..
GENERAL METHOD OF SYNTHESIS
ADD YOUR METHYL AT RIGHT PLACE
As is evident to those skilled in the art, the many of the deacetylase inhibitor compounds of the present invention contain asymmetric carbon atoms. It should be understood, therefore, that the individual stereoisomers are contemplated as being included within the scope of this invention.
The hydroxamate compounds of the present invention can be produced by known organic synthesis methods. For example, the hydroxamate compounds can be produced by reacting methyl 4-formyl cinnamate with tryptamine and then converting the reactant to the hydroxamate compounds. As an example, methyl 4-formyl cinnamate 2, is prepared by acid catalyzed esterification of 4-formylcinnamic acid 3 (Bull. Chem. Soc. Jpn. 1995; 68:2355-2362). An alternate preparation of methyl 4-formyl cinnamate 2 is by a Pd- catalyzed coupling of methyl acrylate 4 with 4-bromobenzaldehyde 5.
CHO
Additional starting materials can be prepared from 4-carboxybenzaldehyde 6, and an exemplary method is illustrated for the preparation of aldehyde 9, shown below. The carboxylic acid in 4-carboxybenzaldehyde 6 can be protected as a silyl ester (e.g., the t- butyldimethylsilyl ester) by treatment with a silyl chloride (e.g., f-butyldimethylsilyl chloride) and a base (e.g. triethylamine) in an appropriate solvent (e.g., dichloromethane). The resulting silyl ester 7 can undergo an olefination reaction (e.g., a Horner-Emmons olefination) with a phosphonate ester (e.g., triethyl 2-phosphonopropionate) in the presence of a base (e.g., sodium hydride) in an appropriate solvent (e.g., tetrahydrofuran (THF)). Treatment of the resulting diester with acid (e.g., aqueous hydrochloric acid) results in the hydrolysis of the silyl ester providing acid 8. Selective reduction of the carboxylic acid of 8 using, for example, borane-dimethylsuflide complex in a solvent (e.g., THF) provides an intermediate alcohol. This intermediate alcohol could be oxidized to aldehyde 9 by a number of known methods, including, but not limited to, Swern oxidation, Dess-Martin periodinane oxidation, Moffatt oxidation and the like.
The aldehyde starting materials 2 or 9 can be reductively aminated to provide secondary or tertiary amines. This is illustrated by the reaction of methyl 4-formyl cinnamate 2 with tryptamine 10 using sodium triacetoxyborohydride (NaBH(OAc)3) as the reducing agent in dichloroethane (DCE) as solvent to provide amine 11. Other reducing agents can be used, e.g., sodium borohydride (NaBH ) and sodium cyanoborohydride (NaBH3CN), in other solvents or solvent mixtures in the presence or absence of acid catalysts (e.g., acetic acid and trifluoroacetic acid). Amine 11 can be converted directly to hydroxamic acid 12 by treatment with 50% aqueous hydroxylamine in a suitable solvent (e.g., THF in the presence of a base, e.g., NaOH). Other methods of hydroxamate formation are known and include reaction of an ester with hydroxylamine hydrochloride and a base (e.g., sodium hydroxide or sodium methoxide) in a suitable solvent or solvent mixture (e.g., methanol, ethanol or methanol/THF).
NOTE ….METHYL SUBSTITUENT ON 10 WILL GIVE YOU PANOBINOSTAT
……………………………….
Journal of Medicinal Chemistry, 2011 , vol. 54, 13 pg. 4694 – 4720
(E)-N-Hydroxy-3-(4-{[2-(2-methyl-1H-indol-3-yl)-ethylamino]-methyl}-phenyl)-acrylamide
lactate
lactate
(34, panobinostat, LBH589)
for str see above link
α-methyl-β-(β-bromoethyl)indole (29) was made according to method reported by Grandberg et al.(2. Grandberg, I. I.; Kost, A. N.; Terent’ev, A. P. Reactions of hydrazine derivatives. XVII. New synthesis of α-methyltryptophol. Zhurnal Obshchei Khimii 1957, 27, 3342–3345. )
The bromide 29 was converted to amine 30 by using similar method used by Sletzinger et al.(3. Sletzinger, M.; Ruyle, W. V.; Waiter, A. G. (Merck & Co., Inc.). Preparation of tryptamine
derivatives. U.S. Patent US 2,995,566, Aug 8, 1961.)
derivatives. U.S. Patent US 2,995,566, Aug 8, 1961.)
To a 500 mL flask, crude 2-methyltryptamine 30 (HPLC purity 75%, 1.74 g, 7.29 mmol) and 3-(4-
formyl-phenyl)-acrylic acid methyl ester 31 (HPLC purity 84%, 1.65 g, 7.28 mmol) were added,
followed by DCM (100 mL) and MeOH (30 mL). The clear solution was stirred at room temp for 30
min, then NaBH3CN (0.439 g, 6.99 mmol) was added in small portions. The reaction mixture was
stirred at room temp overnight. After removal of the solvents, the residue was diluted with DCM and
added saturated NaHCO3 aqueous solution, extracted with DCM twice. The DCM layer was dried
and concentrated, and the resulting residue was purified by flash chromatography (silica, 0–10%
MeOH in DCM) to afford 33 as orange solid (1.52 g, 60%). LC–MS m/z 349.2 ([M + H]+). 33 was
converted to hydroxamic acid 34 according to procedure D (Experimental Section), and the freebase
34 was treated with 1 equiv of lactic acid in MeOH–water (7:3) to form lactic acid salt which was
further recrystallized in MeOH–EtOAc to afford the lactic acid salt of 34as pale yellow solid. LC–MS m/z 350.2 ([M + H − lactate]+).
formyl-phenyl)-acrylic acid methyl ester 31 (HPLC purity 84%, 1.65 g, 7.28 mmol) were added,
followed by DCM (100 mL) and MeOH (30 mL). The clear solution was stirred at room temp for 30
min, then NaBH3CN (0.439 g, 6.99 mmol) was added in small portions. The reaction mixture was
stirred at room temp overnight. After removal of the solvents, the residue was diluted with DCM and
added saturated NaHCO3 aqueous solution, extracted with DCM twice. The DCM layer was dried
and concentrated, and the resulting residue was purified by flash chromatography (silica, 0–10%
MeOH in DCM) to afford 33 as orange solid (1.52 g, 60%). LC–MS m/z 349.2 ([M + H]+). 33 was
converted to hydroxamic acid 34 according to procedure D (Experimental Section), and the freebase
34 was treated with 1 equiv of lactic acid in MeOH–water (7:3) to form lactic acid salt which was
further recrystallized in MeOH–EtOAc to afford the lactic acid salt of 34as pale yellow solid. LC–MS m/z 350.2 ([M + H − lactate]+).
= DELTA
1H NMR (DMSO-d6) 10.72 (s, 1H, NH), 7.54 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 16 Hz, 1H), 7.43 (d, J = 7.8 Hz, 2H), 7.38 (d, J = 7.6 Hz, 1H), 7.22 (d, J = 7.8 Hz, 1H), 6.97 (td, J = 7.8 Hz, 1H), 7.44 (d, J = 15.8 Hz, 1H), 7.22 (t, J = 7.8 Hz, 2H), 7.08 (d, J = 7.8Hz, 2H), 7.01 (t, J = 7.4, 0.9 Hz, 1H), 6.91 (td, J = 7.4, 0.9 Hz, 1H), 6.47 (d, J = 15.2 Hz, 1H), 3.94(q, J = 6.8 Hz, 1H, lactate CH), 3.92 (s, 2H), 2.88 and 2.81 (m, each, 4H, AB system, CH2CH2),2.31 (s, 3H), 1.21 (d, J = 6.8 Hz, 3H).;
13C NMR (DMSO-d6) 176.7 (lactate C=O), 162.7, 139.0,
137.9, 135.2, 134.0, 132.1, 129.1, 128.1, 127.4, 119.9, 119.0, 118.1, 117.2, 110.4, 107.0, 66.0, 51.3,
48.5, 22.9, 20.7, 11.2.
137.9, 135.2, 134.0, 132.1, 129.1, 128.1, 127.4, 119.9, 119.0, 118.1, 117.2, 110.4, 107.0, 66.0, 51.3,
48.5, 22.9, 20.7, 11.2.
…………………………………………..
PANOBINOSTAT DRUG SUBSTANCE SYNTHESIS AND DATA
A flow diagram for the synthesis of LBH589 lactate is provided in FIG. A. A nomenclature reference index of the intermediates is provided below in the Nomenclature Reference Index:
| Nomenclature reference index | |
| Compound | Chemical name |
| 1 | 4-Bromo-benzaldehyde |
| 2 | Methyl acrylate |
| 3 | (2E)-3-(formylphenyl)-2-propenoic acid, methyl ester |
| 4 | 3-[4-[[[2-(2-Methyl-1H-indol-3- |
| yl)ethyl]amino]methyl]phenyl]-2- | |
| propenoic acid, methyl ester, monohydrochloride | |
| 5 | (2E)-N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3- |
| yl)ethyl]amino]methyl]phenyl]-2-propenamide | |
| 6 | 2-hydroxypropanoic acid, compd. with 2(E)-N- |
| hydroxy-3-[4-[[[2-(2-methyl-1H- | |
| indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide | |
| Z3a | 2-Methyl-1H-indole-3-ethanamine |
| Z3b | 5-Chloro-2-pentanone |
| Z3c | Phenylhydrazine |
The manufacture of LBH589 lactate (6) drug substance is via a convergent synthesis; the point of convergence is the condensation of indole-amine Z3a with aldehyde 3.
The synthesis of indole-amine Z3a involves reaction of 5-chloro-2 pentanone (Z3b) with phenylhydrazine (Z3c) in ethanol at reflux (variation of Fischer indole synthesis).
Product isolation is by an extractive work-up followed by crystallization. Preparation of aldehyde 3 is by palladium catalyzed vinylation (Heck-type reaction; Pd(OAc)2/P(o-Tol)3/Bu3N in refluxing CH3CN) of 4-bromo-benzyladehyde (1) with methyl acrylate (2) with product isolation via precipitation from dilute HCl solution. Intermediates Z3a and 3 are then condensed to an imine intermediate, which is reduced using sodium borohydride in methanol below 0° C. (reductive amination). The product indole-ester 4, isolated by precipitation from dilute HCl, is recrystallized from methanol/water, if necessary. The indole ester 4 is converted to crude LBH589 free base 5 via reaction with hydroxylamine and sodium hydroxide in water/methanol below 0° C. The crude LBH589 free base 5 is then purified by recrystallization from hot ethanol/water, if necessary. LBH589 free base 5 is treated with 85% aqueous racemic lactic acid and water at ambient temperature. After seeding, the mixture is heated to approximately 65° C., stirred at this temperature and slowly cooled to 45-50° C. The resulting slurry is filtered and washed with water and dried to afford LBH589 lactate (6).
If necessary the LBH589 lactate 6 may be recrystallised once again from water in the presence of 30 mol % racemic lactic acid. Finally the LBH589 lactate is delumped to give the drug substance. If a rework of the LBH589 lactate drug substance 6 is required, the LBH589 lactate salt is treated with sodium hydroxide in ethanol/water to liberate the LBH589 free base 5 followed by lactate salt formation and delumping as described above.
All starting materials, reagents and solvents used in the synthesis of LBH589 lactate are tested according to internal specifications or are purchased from established suppliers against a certificate of analysis.
EXAMPLE 7 Formation of Monohydrate Lactate Salt
About 40 to 50 mg of N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide free base was suspended in 1 ml of a solvent as listed in Table 7. A stoichiometric amount of lactic acid was subsequently added to the suspension. The mixture was stirred at ambient temperature and when a clear solution formed, stirring continued at 4° C. Solids were collected by filtration and analyzed by XRPD, TGA and 1H-NMR.
| TABLE 7 | |||||
| LOD, % | |||||
| Physical | Crystallinity | (Tdesolvation) | |||
| Solvent | T, ° C. | Appear. | and Form | Tdecomposit. | 1H-NMR |
| IPA | 4 | FFP | excellent | 4.3 (79.3) | — |
| HA | 156.3 | ||||
| Acetone | 4 | FFP | excellent | 4.5 (77.8) | 4.18 (Hbz) |
| HA | 149.5 | ||||
The salt forming reaction in isopropyl alcohol and acetone at 4° C. produced a stoichiometric (1:1) lactate salt, a monohydrate. The salt is crystalline, begins to dehydrate above 77° C., and decomposes above 150° C.
EXAMPLE 18 Formation of Anhydrous Lactate Salt
DL-lactic acid (4.0 g, 85% solution in water, corresponding to 3.4 g pure DL-lactic acid) is diluted with water (27.2 g), and the solution is heated to 90° C. (inner temperature) for 15 hours. The solution is allowed to cool down to room temperature and is used as lactic acid solution for the following salt formation step.
N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide free base (10.0 g) is placed in a 4-necked reaction flask with mechanical stirrer. Demineralized water (110.5 g) is added, and the suspension is heated to 65° C. (inner temperature) within 30 minutes. The DL-lactic acid solution is added to this suspension during 30 min at 65° C. During the addition of the lactate salt solution, the suspension converted into a solution. The addition funnel is rinsed with demineralized water (9.1 g), and the solution is stirred at 65° C. for an additional 30 minutes. The solution is cooled down to 45° C. (inner temperature) and seed crystals (10 mg N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate monohydrate) are added at this temperature. The suspension is cooled down to 33° C. and is stirred for additional 20 hours at this temperature. The suspension is re-heated to 65° C., stirred for 1 hour at this temperature and is cooled to 33° C. within 1 hour. After additional stirring for 3 hours at 33° C., the product is isolated by filtration, and the filter cake is washed with demineralized water (2×20 g). The wet filter-cake is dried in vacuo at 50° C. to obtain the anhydrous N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt as a crystalline product. The product is identical to the monohydrate salt (form HA) in HPLC and in 1H-NMR, with the exception of the integrals of water signals in the 1H-NMR spectra.
In additional salt formation experiments carried out according to the procedure described above, the product solution was filtered at 65° C. before cooling to 45° C., seeding and crystallization. In all cases, form A (anhydrate form) was obtained as product.
EXAMPLE 19 Formation of Anhydrous Lactate Salt
DL-lactic acid (2.0 g, 85% solution in water, corresponding to 1.7 g pure DL-lactic acid) is diluted with water (13.6 g), and the solution is heated to 90° C. (inner temperature) for 15 hours. The solution was allowed to cool down to room temperature and is used as lactic acid solution for the following salt formation step.
N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide free base (5.0 g) is placed in a 4-necked reaction flask with mechanical stirrer. Demineralized water (54.85 g) is added, and the suspension is heated to 48° C. (inner temperature) within 30 minutes. The DL-lactic acid solution is added to this suspension during 30 minutes at 48° C. A solution is formed. Seed crystals are added (as a suspension of 5 mg N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt, anhydrate form A, in 0.25 g of water) and stirring is continued for 2 additional hours at 48° C. The temperature is raised to 65° C. (inner temperature) within 30 minutes, and the suspension is stirred for additional 2.5 hours at this temperature. Then the temperature is cooled down to 48° C. within 2 hours, and stirring is continued at this temperature for additional 22 hours. The product is isolated by filtration and the filter cake is washed with demineralized water (2×10 g). The wet filter-cake is dried in vacuo at 50° C. to obtain anhydrous N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt (form A) as a crystalline product.
EXAMPLE 20 Conversion of Monohydrate Lactate Salt to Anhydrous Lactate Salt
DL-lactic acid (0.59 g, 85% solution in water, corresponding to 0.5 g pure DL-lactic acid) is diluted with water (4.1 g), and the solution is heated to 90° C. (inner temperature) for 15 hours. The solution is allowed to cool down to room temperature and is used as lactic acid solution for the following salt formation step.
10 g of N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt monohydrate is placed in a 4-necked reaction flask. Water (110.9 g) is added, followed by the addition of the lactic acid solution. The addition funnel of the lactic acid is rinsed with water (15.65 g). The suspension is heated to 82° C. (inner temperature) to obtain a solution. The solution is stirred for 15 minutes at 82° C. and is hot filtered into another reaction flask to obtain a clear solution. The temperature is cooled down to 50° C., and seed crystals are added (as a suspension of 10 mg N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt, anhydrate form, in 0.5 g of water). The temperature is cooled down to 33° C. and stirring is continued for additional 19 hours at this temperature. The formed suspension is heated again to 65° C. (inner temperature) within 45 minutes, stirred at 65° C. for 1 hour and cooled down to 33° C. within 1 hour. After stirring at 33° C. for additional 3 hours, the product is isolated by filtration and the wet filter cake is washed with water (50 g). The product is dried in vacuo at 50° C. to obtain crystalline anhydrous N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl) ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt (form A).
EXAMPLE 21 Formation of Anhydrous Lactate Salt
DL-lactic acid (8.0 g, 85% solution in water, corresponding to 6.8 g pure DL-lactic acid) was diluted with water (54.4 g), and the solution was heated to 90° C. (inner temperature) for 15 hours. The solution was allowed to cool down to room temperature and was used as lactic acid solution for the following salt formation step.
N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide (20 g) is placed in a 1 L glass reactor, and ethanol/water (209.4 g of a 1:1 w/w mixture) is added. The light yellow suspension is heated to 60° C. (inner temperature) within 30 minutes, and the lactic acid solution is added during 30 minutes at this temperature. The addition funnel is rinsed with water (10 g). The solution is cooled to 38° C. within 2 hours, and seed crystals (20 mg of N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt, anhydrate form) are added at 38° C. After stirring at 38° C. for additional 2 hours, the mixture is cooled down to 25° C. within 6 hours. Cooling is continued from 25° C. to 10° C. within 5 hours, from 10° C. to 5° C. within 4 hours and from 5° C. to 2° C. within 1 hour. The suspension is stirred for additional 2 hours at 2° C., and the product is isolated by filtration. The wet filter cake is washed with water (2×30 g), and the product is dried in vacuo at 45° C. to obtain crystalline anhydrous N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide lactate salt (form A).
EXAMPLE 28 Formation of Lactate Monohydrate Salt
3.67 g (10 mmol) of the free base monohydrate (N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl) ethyl]amino]methyl]phenyl]-2E-2-propenamide) and 75 ml of acetone were charged in a 250 ml 3-neck flask equipped with a magnetic stirrer and an addition funnel. To the stirred suspension were added dropwise 10 ml of 1 M lactic acid in water (10 mmol) dissolved in 20 ml acetone, affording a clear solution. Stirring continued at ambient and a white solid precipitated out after approximately 1 hour. The mixture was cooled in an ice bath and stirred for an additional hour. The white solid was recovered by filtration and washed once with cold acetone (15 ml). It was subsequently dried under vacuum to yield 3.94 g of the lactate monohydrate salt of N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide (86.2%).
References
- Revill, P; Mealy, N; Serradell, N; Bolos, J; Rosa, E (2007). “Panobinostat”. Drugs of the Future 32 (4): 315. doi:10.1358/dof.2007.032.04.1094476. ISSN 0377-8282.
- Table 3: Select epigenetic inhibitors in various stages of development from Mack, G. S. (2010). “To selectivity and beyond”. Nature Biotechnology 28 (12): 1259–1266.doi:10.1038/nbt.1724. PMID 21139608. edit
- ClinicalTrials.gov NCT00425555 Study of Oral LBH589 in Adult Patients With Refractory Cutaneous T-Cell Lymphoma
- ClinicalTrials.gov: LBH-589
- Prince, HM; M Bishton (2009). “Panobinostat (LBH589): a novel pan-deacetylase inhibitor with activity in T cell lymphoma”. Hematology Meeting Reports (Parkville, Australia: Peter MacCallum Cancer Centre and University of Melbourne) 3 (1): 33–38.
- Simons, J (27 April 2013). “Scientists on brink of HIV cure”. The Telegraph.
- ClinicalTrials.gov NCT01680094 Safety and Effect of The HDAC Inhibitor Panobinostat on HIV-1 Expression in Patients on Suppressive HAART (CLEAR)
- Mayo Clinic Researchers Formulate Treatment Combination Lethal To Pancreatic Cancer Cells
- Garbes, L; Riessland, M; Hölker, I; Heller, R; Hauke, J; Tränkle, Ch; Coras, R; Blümcke, I; Hahnen, E; Wirth, B (2009). “LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate”. Human Molecular Genetics 18 (19): 3645–3658. doi:10.1093/hmg/ddp313.PMID 19584083.
- Tate, CR; Rhodes, LV; Segar, HC; Driver, JL; Pounder, FN; Burow, ME; and Collins-Burow, BM (2012). “Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat”. Breast Cancer Research 14 (3).
- TA Rasmussen, et al. Comparison of HDAC inhibitors in clinical development: Effect on HIV production in latently infected cells and T-cell activation. Human Vaccines & Immunotherapeutics 9:5, 1-9, May 2013.
- Drugs of the Future 32(4): 315-322 (2007)
- WO 2002022577…
- WO 2007146718
- WO 2013110280
- WO 2010009285
- WO 2010009280
- WO 2005013958
- WO 2004103358
- WO 2003048774…
- Journal of Medicinal Chemistry, 2011 , vol. 54, 13 pg. 4694 – 4720
23009203 11-26-2012 Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth. Journal of medicinal chemistry 21634430 7-14-2011 Discovery of (2E)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1H-benzimidazol-5-yl}-N-hydroxyacrylamide (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile. Journal of medicinal chemistry 21417419 4-28-2011 Discovery, synthesis, and pharmacological evaluation of spiropiperidine hydroxamic acid based derivatives as structurally novel histone deacetylase (HDAC) inhibitors. Journal of medicinal chemistry 19317450 4-23-2009 Identification and characterization of small molecule inhibitors of a class I histone deacetylase from Plasmodium falciparum. Journal of medicinal chemistry 15650931 1-1-2005 The American Society of Hematology–46th Annual Meeting and Exposition. HDAC, Flt and farnesyl transferase inhibitors. IDrugs : the investigational drugs journal US7989639 8-3-2011 PROCESS FOR MAKING SALTS OF N-HYDROXY-3-[4-[[[2-(2-METHYL-1H-INDOL-3-YL)ETHYL]AMINO]METHYL]PHENYL]-2E-2-PROPENAMIDE US2010286409 11-12-2010 SALTS OF N-HYDROXY-3-[4-[[[2-(2-METHYL-1H-INDOL-3-YL)ETHYL]AMINO]METHYL]PHENYL]-2E-2-PROPENAMIDE US2010179208 7-16-2010 Use of HDAC Inhibitors for the Treatment of Bone Destruction US2010160257 6-25-2010 USE OF HDAC INHIBITORS FOR THE TREATMENT OF MYELOMA US2010137398 6-4-2010 USE OF HDAC INHIBITORS FOR THE TREATMENT OF GASTROINTESTINAL CANCERS US2009306405 12-11-2009 PROCESS FOR MAKING N-HYDROXY-3-[4-[[[2-(2-METHYL-1H-INDOL-3-YL)ETHYL]AMINO]METHYL]PHENYL]-2E-2-PROPENAMIDE AND STARTING MATERIALS THEREFOR US2009281159 11-13-2009 USE OF HDAC INHIBITORS FOR THE TREATMENT OF LYMPHOMAS US2009264439 10-23-2009 Combination of a) N–4-(3-pyridyl)-2-pyrimidine-amine and b) a histone deacetylase inhibitor for the treatment of leukemia US2009197936 8-7-2009 SALTS OF N-HYDROXY-3-[4-[[[2-(2-METHYL-1H-INDOL-3-YL)ETHYL]AMINO]METHYL]PHENYL]-2E-2-PROPENAMIDE US2009012066 1-9-2009 Method of Use of Deacetylase Inhibitors
| US2008319045 | 12-26-2008 | Combination of Histone Deacetylase Inhibitors and Radiation |
| US2008221126 | 9-12-2008 | Use of Hdac Inhibitors for the Treatment of Myeloma |
| US2008176849 | 7-25-2008 | DEACETYLASE INHIBITORS |
| US2006189674 | 8-25-2006 | Deacetylase inhibitors |
| US7067551 | 6-28-2006 | Deacetylase inhibitors |
| US2006100140 | 5-12-2006 | Combination of a) n-{5-[4-(4-methyl-piperazino-methyl)-benzoylamido]2-methylphenyl}-4- (3-pyridyl)-2-pyrimidine-amine and b) a histone deacetylase inhibitor for the treatment of leukemia |
| US6833384 | 12-22-2004 | Deacetylase inhibitors |
| US6552065 | 4-23-2003 | Deacetylase inhibitors |
| GB776693A | Title not available | |||
| GB891413A | Title not available | |||
| GB2185020A | Title not available | |||
| WO2002022577A2 | Aug 30, 2001 | Mar 21, 2002 | Kenneth Walter Bair | Hydroxamate derivatives useful as deacetylase inhibitors |
| WO2003016307A1 | Aug 6, 2002 | Aug 19, 1993 | Jolie Anne Bastian | β3 ADRENERGIC AGONISTS |
| WO2003039599A1 | Nov 5, 2002 | May 15, 2003 | Ying-Nan Pan Chen | Cyclooxygenase-2 inhibitor/histone deacetylase inhibitor combination |
| WO2005105740A2 | Apr 26, 2005 | Nov 10, 2005 | Serguei Fine | Preparation of tegaserod and tegaserod maleate |
| WO2006021397A1 | Aug 22, 2005 | Mar 2, 2006 | Recordati Ireland Ltd | Lercanidipine salts |
................................................................................
2 BELINOSTAT
Belinostat (PXD101)
PHASE 2, FAST TRACK FDA , ORPHAN STATUS
- PDX101
- PX 105684
- PXD-101
- PXD101
- UNII-F4H96P17NZ
Belinostat (PXD101) is a novel HDAC inhibitor with IC50 of 27 nM, with activity demonstrated in cisplatin-resistant tumors.
Belinostat inhibits the growth of tumor cells (A2780, HCT116, HT29, WIL, CALU-3, MCF7, PC3 and HS852) with IC50 from 0.2-0.66 μM. PD101 shows low activity in A2780/cp70 and 2780AD cells. Belinostat inhibits bladder cancer cell growth, especially in 5637 cells, which shows accumulation of G0-G1 phase, decrease in S phase, and increase in G2-M phase. Belinostat also shows enhanced tubulin acetylation in ovarian cancer cell lines. A recent study shows that Belinostat activates protein kinase A in a TGF-β signaling-dependent mechanism and decreases survivin mRNA.
| MW 318.07 | |
| MF | C15H14N2O4S |
414864-00-9 cas no
866323-14-0
(2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]acrylamide
A novel HDAC inhibitor
…………………………
Belinostat (PXD101) is experimental drug candidate under development byTopoTarget for the treatment of hematological malignancies and solid tumors. It is a histone deacetylase inhibitor.[1]
A hydroxamate-type inhibitor of histone deacetylase.
NCI: A novel hydroxamic acid-type histone deacetylase (HDAC) inhibitor with antineoplastic activity. Belinostat targets HDAC enzymes, thereby inhibiting tumor cell proliferation, inducing apoptosis, promoting cellular differentiation, and inhibiting angiogenesis. This agent may sensitize drug-resistant tumor cells to other antineoplastic agents, possibly through a mechanism involving the down-regulation of thymidylate synthase
In 2007 preliminary results were released from the Phase II clinical trial of intravenous belinostat in combination with carboplatin and paclitaxel for relapsedovarian cancer.[2] Final results in late 2009 of a phase II trial for T cell lymphomawere encouraging.[3] Belinostat has been granted orphan drug and fast trackdesignation by the FDA.[4]
The study of inhibitors of histone deacetylases indicates that these enzymes play an important role in cell proliferation and differentiation. The inhibitor Trichostatin A (TSA) (Yoshida et al., 1990a) causes cell cycle arrest at both G1 and G2 phases (Yoshida and Beppu, 1988), reverts the transformed phenotype of different cell lines, and induces differentiation of Friend leukaemia cells and others (Yoshida et al., 1990b). TSA (and SAHA) have been reported to inhibit cell growth, induce terminal differentiation, and prevent the formation of tumours in mice (Finnin et al., 1999).
Trichostatin A (TSA)
Suberoylanilide Hydroxamic Acid (SAHA)
Cell cycle arrest by TSA correlates with an increased expression of gelsolin (Hoshikawa et al., 1994), an actin regulatory protein that is down regulated in malignant breast cancer (Mielnicki et al., 1999). Similar effects on cell cycle and differentiation have been observed with a number of deacetylase inhibitors (Kim et al., 1999). Trichostatin A has also been reported to be useful in the treatment of fibrosis, e.g., liver fibrosis and liver cirrhosis. See, e.g., Geerts et al., 1998.
Recently, certain compounds that induce differentiation have been reported to inhibit histone deacetylases. Several experimental antitumour compounds, such as trichostatin A (TSA), trapoxin, suberoylanilide hydroxamic acid (SAHA), and phenylbutyrate have been reported to act, at least in part, by inhibiting histone deacetylase (see, e.g., Yoshida et al., 1990; Richon et al., 1998; Kijima et al., 1993). Additionally, diallyl sulfide and related molecules (see, e.g., Lea et al., 1999), oxamflatin (see, e.g., Kim et al., 1999), MS-27-275, a synthetic benzamide derivative (see, e.g., Saito et al., 1999; Suzuki et al., 1999; note that MS-27-275 was later re-named as MS-275), butyrate derivatives (see, e.g., Lea and Tulsyan, 1995), FR901228 (see, e.g., Nokajima et al., 1998), depudecin (see, e.g., Kwon et al., 1998), and m-carboxycinnamic acid bishydroxamide (see, e.g., Richon et al., 1998) have been reported to inhibit histone deacetylases. In vitro, some of these compounds are reported to inhibit the growth of fibroblast cells by causing cell cycle arrest in the G1 and G2 phases, and can lead to the terminal differentiation and loss of transforming potential of a variety of transformed cell lines (see, e.g., Richon et al, 1996; Kim et al., 1999; Yoshida et al., 1995; Yoshida & Beppu, 1988). In vivo, phenybutyrate is reported to be effective in the treatment of acute promyelocytic leukemia in conjunction with retinoic acid (see, e.g., Warrell et al., 1998). SAHA is reported to be effective in preventing the formation of mammary tumours in rats, and lung tumours in mice (see, e.g., Desai et al., 1999).
The clear involvement of HDACs in the control of cell proliferation and differentiation suggest that aberrant HDAC activity may play a role in cancer. The most direct demonstration that deacetylases contribute to cancer development comes from the analysis of different acute promyelocytic leukaemias (APL). In most APL patients, a translocation of chromosomes 15 and 17 (t(15;17)) results in the expression of a fusion protein containing the N-terminal portion of PML gene product linked to most of RARσ (retinoic acid receptor). In some cases, a different translocation (t(11 ;17)) causes the fusion between the zinc finger protein PLZF and RARα. In the absence of ligand, the wild type RARα represses target genes by tethering HDAC repressor complexes to the promoter DNA. During normal hematopoiesis, retinoic acid (RA) binds RARα and displaces the repressor complex, allowing expression of genes implicated in myeloid differentiation. The RARα fusion proteins occurring in APL patients are no longer responsive to physiological levels of RA and they interfere with the expression of the RA- inducible genes that promote myeloid differentiation. This results in a clonal expansion of promyelocytic cells and development of leukaemia. In vitro experiments have shown that TSA is capable of restoring RA-responsiveness to the fusion RARα proteins and of allowing myeloid differentiation. These results establish a link between HDACs and oncogenesis and suggest that HDACs are potential targets for pharmaceutical intervention in APL patients. (See, for example, Kitamura et al., 2000; David et al., 1998; Lin et al., 1998).
Furthermore, different lines of evidence suggest that HDACs may be important therapeutic targets in other types of cancer. Cell lines derived from many different cancers (prostate, coloreetal, breast, neuronal, hepatic) are induced to differentiate by HDAC inhibitors (Yoshida and Horinouchi, 1999). A number of HDAC inhibitors have been studied in animal models of cancer. They reduce tumour growth and prolong the lifespan of mice bearing different types of transplanted tumours, including melanoma, leukaemia, colon, lung and gastric carcinomas, etc. (Ueda et al., 1994; Kim et al., 1999).
Psoriasis is a common chronic disfiguring skin disease which is characterised by well-demarcated, red, hardened scaly plaques: these may be limited or widespread. The prevalence rate of psoriasis is approximately 2%, i.e., 12.5 million sufferers in the triad countries (US/Europe/Japan). While the disease is rarely fatal, it clearly has serious detrimental effects upon the quality of life of the patient: this is further compounded by the lack of effective therapies. Present treatments are either ineffective, cosmetically unacceptable, or possess undesired side effects. There is therefore a large unmet clinical need for effective and safe drugs for this condition. Psoriasis is a disease of complex etiology. Whilst there is clearly a genetic component, with a number of gene loci being involved, there are also undefined environmental triggers. Whatever the ultimate cause of psoriasis, at the cellular level, it is characterised by local T-cell mediated inflammation, by keratinocyte hyperproliferation, and by localised angiogenesis. These are all processes in which histone deacetylases have been implicated (see, e.g., Saunders et al., 1999; Bernhard et al, 1999; Takahashi et al, 1996; Kim et al , 2001 ). Therefore HDAC inhibitors may be of use in therapy for psoriasis. Candidate drugs may be screened, for example, using proliferation assays with T-cells and/or keratinocytes.
………………………………………………………………………..
PXD101/Belinostat®
(E)-N-hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide, also known as PXD101 and Belinostat®, shown below, is a well known histone deacetylate (HDAC) inhibitor. It is being developed for treatment of a range of disorders mediated by HDAC, including proliferative conditions (such as cancer and psoriasis), malaria, etc.
PXD101 was first described in WO 02/30879 A2. That document describes a multi-step method of synthesis which may conveniently be illustrated by the following scheme.
…………………………………..
GENERAL SYNTHESIS
IGNORE 10
ENTRY 45 IS BELINOSTAT
Scheme 1
By using amines instead of aniline, the corresponding products may be obtained. The use of aniline, 4-methoxyaniline, 4-methylaniline, 4-bromoaniline, 4-chloroaniline, 4-benzylamine, and 4-phenethyamine, among others, is described in the Examples below.
In another method, a suitable amino acid (e.g., ω-amino acid) having a protected carboxylic acid (e.g., as an ester) and an unprotected amino group is reacted with a sulfonyl chloride compound (e.g., RSO2CI) to give the corresponding sulfonamide having a protected carboxylic acid. The protected carboxylic acid is then deprotected using base to give the free carboxylic acid, which is then reacted with, for example, hydroxylamine 2-chlorotrityl resin followed by acid (e.g., trifluoroacetic acid), to give the desired carbamic acid.
One example of this approach is illustrated below, in Scheme 2, wherein the reaction conditions are as follows: (i) RSO2CI, pyridine, DCM, room temperature, 12 hours; (ii) 1 M LiOH or 1 M NaOH, dioxane, room temperature, 3-48 hours; (iii) hydroxylamine 2-chlorotrityl resin, HOAt, HATU, DIPEA, DCM, room temperature, 16 hours; and (iv) TFA/DCM (5:95, v/v), room temperature, 1.5 hours.
Scheme 2
Additional methods for the synthesis of compounds of the present invention are illustrated below and are exemplified in the examples below.
Scheme 3A
Scheme 3B
Scheme 4
Scheme 8
Scheme 9
……………………………………………………………………..
SYNTHESIS
Example 1
3-Formylbenzenesulfonic acid, sodium salt (1)
Oleum (5 ml) was placed in a reaction vessel and benzaldehyde (2.00 g, 18.84 mmol) was slowly added not exceeding the temperature of the reaction mixture more than 30°C. The obtained solution was stirred at 40°C for ten hours and at ambient temperature overnight. The reaction mixture was poured into ice and extracted with ethyl acetate. The aqueous phase was treated with CaC03 until the evolution of C02 ceased (pH~6-7), then the precipitated CaSO4was filtered off and washed with water. The filtrate was treated with Na2CO3 until the pH of the reaction medium increased to pH 8, obtained CaCO3 was filtered off and water solution was evaporated in vacuum. The residue was washed with methanol, the washings were evaporated and the residue was dried in desiccator over P2Oβ affording the title compound (2.00 g, 51%). 1H NMR (D20), δ: 7.56-8.40 (4H, m); 10.04 ppm (1 H, s).
Example 2 3-(3-Sulfophenyl)acrylic acid methyl ester, sodium salt (2)
Sodium salt of 3-formylbenzenesulfonic acid (1) (1.00 g, 4.80 mmol), potassium carbonate (1.32 g, 9.56 mmol), trimethyl phosphonoacetate (1.05 g, 5.77 mmol) and water (2 ml) were stirred at ambient temperature for 30 min., precipitated solid was filtered and washed with methanol. The filtrate was evaporated and the title compound (2) was obtained as a white solid (0.70 g, 55%). 1H NMR (DMSO- dβl HMDSO), δ: 3.68 (3H, s); 6.51 (1 H, d, J=16.0 Hz); 7.30-7.88 (5H, m).
Example 3 3-(3-Chlorosulfonylphenyl)acrylic acid methyl ester (3)
To the sodium salt of 3-(3-sulfophenyl)acrylic acid methyl ester (2) (0.670 g, 2.53 mmol) benzene (2 ml), thionyl chloride (1.508 g, 0.9 ml, 12.67 mmol) and 3 drops of dimethylformamide were added and the resultant suspension was stirred at reflux for one hour. The reaction mixture was evaporated, the residue was dissolved in benzene (3 ml), filtered and the filtrate was evaporated to give the title compound (0.6’40 g, 97%).
Example 4 3-(3-Phenylsulfamoylphenyl)acrylic acid methyl ester (4a)
A solution of 3-(3-chlorosulfonylphenyl)acrylic acid methyl ester (3) (0.640 g, 2.45 mmol) in dichloromethane (2 ml) was added to a mixture of aniline (0.465 g, 4.99 mmol) and pyridine (1 ml), and the resultant solution was stirred at 50°C for one hour. The reaction mixture was evaporated and the residue was partitioned between ethyl acetate and 10% HCI. The organic layer was washed successively with water, saturated NaCl, and dried (Na2S0 ). The solvent was removed and the residue was chromatographed on silica gel with chloroform-ethyl acetate (7:1 , v/v) as eluent. The obtained product was washed with diethyl ether to give the title compound (0.226 g, 29%). 1H NMR (CDCI3, HMDSO), δ: 3.72 (3H, s); 6.34 (1H, d, J=16.0 Hz); 6.68 (1 H, br s); 6.92-7.89 (10H, m).
Example 5 3-(3-Phenylsulfamoylphenyl)acrylic acid (5a)
3-(3-Phenylsulfamoylphenyl)acrylic acid methyl ester (4a) (0.220 g, 0.69 mmol) was dissolved in methanol (3 ml), 1N NaOH (2.08 ml, 2.08 mmol) was added and the resultant solution was stirred at ambient temperature overnight. The reaction mixture was partitioned between ethyl acetate and water. The aqueous layer was acidified with 10% HCI and stirred for 30 min. The precipitated solid was filtered, washed with water and dried in desiccator over P2Os to give the title compound as a white solid (0.173 g, 82%). Example 6 3-(3-Phenylsulfamoylphenyl)acryloyl chloride (6a)
To a suspension of 3-(3-phenylsulfamoylphenyl)acrylic acid (5a) (0.173 g, 0.57 mmol) in dichloromethane (2.3 ml) oxalyl chloride (0.17 ml, 1.95 mmol) and one drop of dimethylformamide were added. The reaction mixture was stirred at 40°C for one hour and concentrated under reduced pressure to give crude title compound (0.185 g).
Example 7
N-Hydroxy-3-(3-phenylsulfamoylphenyl)acrylamide (7a) (PX105684) BELINOSTAT
To a suspension of hydroxylamine hydrochloride (0.200 g, 2.87 mmol) in tetrahydrofuran (3.5 ml) a saturated NaHCOβ solution (2.5 ml) was added and the resultant mixture was stirred at ambient temperature for 10 min. To the reaction mixture a 3-(3-phenylsulfamoylphenyl)acryloyl chloride (6a) (0.185 g) solution in tetrahydrofuran (2.3 ml) was added and stirred at ambient temperature for one hour. The reaction mixture was partitioned between ethyl acetate and 2N HCI. The organic layer was washed successively with water and saturated NaCl, the solvent was removed and the residue was washed with acetonitrile and diethyl ether.
The title compound was obtained as a white solid (0.066 g, 36%), m.p. 172°C. BELINOSTAT
1H NMR (DMSO-d6, HMDSO), δ: 6.49 (1 H, d, J=16.0 Hz); 7.18-8.05 (10H, m); 9.16 (1 H, br s); 10.34 (1 H, s); 10.85 ppm (1 H, br s).
HPLC analysis on Symmetry C18column: impurities 4% (column size 3.9×150 mm; mobile phase acetonitrile – 0.1 M phosphate buffer (pH 2.5), 40:60; sample concentration 1 mg/ml; flow rate 0.8 ml/ min; detector UV 220 nm).
Anal. Calcd for C15Hι4N204S, %: C 56.59, H 4.43, N 8.80. Found, %: C 56.28, H 4.44, N 8.56.
……………………………………………………………………….
SYNTHESIS
US20100286279

…………………………………………………….
SYNTHESIS AND SPECTRAL DATA
Journal of Medicinal Chemistry, 2011 , vol. 54, 13 pg. 4694 – 4720
(E)-N-Hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide (28, belinostat, PXD101).
The methyl ester (27) (8.0 g) was prepared according to reported synthetic route,
(Watkins, C. J.; Romero-Martin, M.-R.; Moore, K. G.; Ritchie, J.; Finn, P. W.; Kalvinsh, I.;
Loza, E.; Dikvoska, K.; Gailite, V.; Vorona, M.; Piskunova, I.; Starchenkov, I.; Harris, C. J.;
Duffy, J. E. S. Carbamic acid compounds comprising a sulfonamide linkage as HDAC
inhibitors. PCT Int. Appl. WO200230879A2, April 18, 2002.)
but using procedure D (Experimental Section) or method described for 26 to convert the methyl ester to crude
hydroxamic acid which was further purified by chromatography (silica, MeOH/DCM = 1:10) to
afford 28 (PXD101) as off-white or pale yellow powder (2.5 g, 31%).
Loza, E.; Dikvoska, K.; Gailite, V.; Vorona, M.; Piskunova, I.; Starchenkov, I.; Harris, C. J.;
Duffy, J. E. S. Carbamic acid compounds comprising a sulfonamide linkage as HDAC
inhibitors. PCT Int. Appl. WO200230879A2, April 18, 2002.)
but using procedure D (Experimental Section) or method described for 26 to convert the methyl ester to crude
hydroxamic acid which was further purified by chromatography (silica, MeOH/DCM = 1:10) to
afford 28 (PXD101) as off-white or pale yellow powder (2.5 g, 31%).
LC–MS m/z 319.0 ([M +H]+).
1H NMR (DMSO-d6) 12–9 (very broad, 2H), 7.90 (s, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.70 (d, J
= 7.8 Hz, 1H), 7.56 (t, J = 7.8 Hz, 1H), 7.44 (d, J = 15.8 Hz, 1H), 7.22 (t, J = 7.8 Hz, 2H), 7.08 (d,
J = 7.8 Hz, 2H), 7.01 (t, J = 7.3 Hz, 1H), 6.50 (d, J = 15.8 Hz, 1H);
J = 7.8 Hz, 2H), 7.01 (t, J = 7.3 Hz, 1H), 6.50 (d, J = 15.8 Hz, 1H);
13C NMR (DMSO-d6) 162.1,
140.6, 138.0, 136.5, 135.9, 131.8, 130.0, 129.2, 127.1, 124.8, 124.1, 121.3, 120.4.
140.6, 138.0, 136.5, 135.9, 131.8, 130.0, 129.2, 127.1, 124.8, 124.1, 121.3, 120.4.
Anal.
(C15H14N2O4S) C, H, N
(C15H14N2O4S) C, H, N
………………………………………………..
SYNTHESIS
PXDIOI / Belinostat®
(E)-N-hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide, also known as PXD101 and Belinostat®, shown below, is a well known histone deacetylate (HDAC) inhibitor. It is being developed for treatment of a range of disorders mediated by HDAC, including proliferative conditions (such as cancer and psoriasis), malaria, etc.
PXD101 was first described in WO 02/30879 A2. That document describes a multi-step method of synthesis which may conveniently be illustrated by the following scheme.
Scheme 1
Not isolated
ed on (A)
on (D)
d on (H)
There is a need for alternative methods for the synthesis of PXD101 and related compounds for example, methods which are simpler and/or employ fewer steps and/or permit higher yields and/or higher purity product.
Scheme 5
DMAP, toluene
Synthesis 1 3-Bromo-N-phenyl-benzenesulfonamide (3)
To a 30 gallon (-136 L) reactor was charged aniline (2) (4.01 kg; 93.13 g/mol; 43 mol), toluene (25 L), and 4-(dimethylamino)pyridine (DMAP) (12 g), and the mixture was heated to 50-600C. 3-Bromobenzenesulfonyl chloride (1) (5 kg; 255.52 g/mol; 19.6 mol) was charged into the reactor over 30 minutes at 50-600C and progress of the reaction was monitored by HPLC. After 19 hours, toluene (5 L) was added due to losses overnight through the vent line and the reaction was deemed to be complete with no compound (1) being detected by HPLC. The reaction mixture was diluted with toluene (10 L) and then quenched with 2 M aqueous hydrochloric acid (20 L). The organic and aqueous layers were separated, the aqueous layer was discarded, and the organic layer was washed with water (20 L), and then 5% (w/w) sodium bicarbonate solution (20 L), while maintaining the batch temperature at 45-55°C. The batch was then used in the next synthesis.
Synthesis 2 (E)-3-(3-Phenylsulfamoyl-phenyl)-acrylic acid ethyl ester (5)
To the batch containing 3-bromo-N-phenyl-benzenesulfonamide (3) (the treated organic layer obtained in the previous synthesis) was added triethylamine (2.97 kg; 101.19 g/mol; 29.4 mol), tri(o-tolyl)phosphine (119 g; 304.37 g/mol; 0.4 mol), and palladium (II) acetate (44 g; 224.51 g/mol; 0.2 mol), and the resulting mixture was degassed four times with a vacuum/nitrogen purge at 45-55°C. Catalytic palladium (0) was formed in situ. The batch was then heated to 80-900C and ethyl acrylate (4) (2.16 kg; 100.12 g/mol; 21.6 mol) was slowly added over 2.75 hours. The batch was sampled after a further 2 hours and was deemed to be complete with no compound (3) being detected by HPLC. The batch was cooled to 45-55°C and for convenience was left at this temperature overnight.
The batch was then reduced in volume under vacuum to 20-25 L, at a batch temperature of 45-55°C, and ethyl acetate (20 L) was added. The batch was filtered and the residue washed with ethyl acetate (3.5 L). The residue was discarded and the filtrates were sent to a 100 gallon (-454 L) reactor, which had been pre-heated to 600C. The 30 gallon (-136 L) reactor was then cleaned to remove any residual Pd, while the batch in the 100 gallon (-454 L) reactor was washed with 2 M aqueous hydrochloric acid and water at 45-55°C. Once the washes were complete and the 30 gallon (-136 L) reactor was clean, the batch was transferred from the 100 gallon (-454 L) reactor back to the 30 gallon (-136 L) reactor and the solvent was swapped under vacuum from ethyl acetate/toluene to toluene while maintaining a batch temperature of 45-55°C (the volume was reduced to 20-25 L). At this point, the batch had precipitated and heptanes (10 L) were added to re-dissolve it. The batch was then cooled to 0-100C and held at this temperature over the weekend in order to precipitate the product. The batch was filtered and the residue was washed with heptanes (5 L). A sample of the wet-cake was taken for Pd analysis. The Pd content of the crude product (5) was determined to be 12.9 ppm.
The wet-cake was then charged back into the 30 gallon (-136 L) reactor along with ethyl acetate (50 L) and heated to 40-500C in order to obtain a solution. A sparkler filter loaded with 12 impregnated Darco G60® carbon pads was then connected to the reactor and the solution was pumped around in a loop through the sparkler filter. After 1 hour, a sample was taken and evaporated to dryness and analysed for Pd content. The amount of Pd was found to be 1.4 ppm. A second sample was taken after 2 hours and evaporated to dryness and analysed for Pd content. The amount of Pd had been reduced to 0.6 ppm. The batch was blown back into the reactor and held at 40-500C overnight before the solvent was swapped under vacuum from ethyl acetate to toluene while maintaining a batch temperature of 45-55°C (the volume was reduced to 20-25 L). At this point, the batch had precipitated and heptanes (10 L) were added to re-dissolve it and the batch was cooled to 0-100C and held at this temperature overnight in order to precipitate the product. The batch was filtered and the residue was washed with heptanes (5 L). The filtrate was discarded and the residue was dried at 45-55°C under vacuum for 25 hours. A first lot of the title compound (5) was obtained as an off-white solid (4.48 kg, 69% overall yield from 3-bromobenzenesulfonyl chloride (1)) with a Pd content of 0.4 ppm and a purity of 99.22% (AUC) by HPLC.
Synthesis 3 (E)-3-(3-Phenylsulfamoyl-phenyl)-acrvlic acid (6)
To the 30 gallon (-136 L) reactor was charged the (E)-3-(3-phenylsulfamoyl-phenyl)- acrylic acid ethyl ester (5) (4.48 kg; 331.39 g/mol; 13.5 mol) along with 2 M aqueous sodium hydroxide (17.76 L; -35 mol). The mixture was heated to 40-50°C and held at this temperature for 2 hours before sampling, at which point the reaction was deemed to be complete with no compound (5) being detected by HPLC. The batch was adjusted to pH 2.2 using 1 M aqueous hydrochloric acid while maintaining the batch temperature between 40-500C. The product had precipitated and the batch was cooled to 20-300C and held at this temperature for 1 hour before filtering and washing the cake with water (8.9 L). The filtrate was discarded. The batch was allowed to condition on the filter overnight before being charged back into the reactor and slurried in water (44.4 L) at 40-500C for 2 hours. The batch was cooled to 15-20°C, held for 1 hour, and then filtered and the residue washed with water (8.9 L). The filtrate was discarded. The crude title compound (6) was transferred to an oven for drying at 45-55°C under vacuum with a slight nitrogen bleed for 5 days (this was done for convenience) to give a white solid (3.93 kg, 97% yield). The moisture content of the crude material was measured using Karl Fischer (KF) titration and found to be <0.1% (w/w). To the 30 gallon (-136 L) reactor was charged the crude compound (6) along with acetonitrile (47.2 L). The batch was heated to reflux (about 80°C) and held at reflux for 2 hours before cooling to 0-10°C and holding at this temperature overnight in order to precipitate the product. The batch was filtered and the residue was washed with cold acetonitrile (7.9 L). The filtrate was discarded and the residue was dried under vacuum at 45-55°C for 21.5 hours. The title compound (6) was obtained as a fluffy white solid (3.37 kg, 84% yield with respect to compound (5)) with a purity of 99.89% (AUC) by HPLC.
Synthesis 4 (E)-N-Hvdroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide (PXD101) BELINOSTAT
To the 30 gallon (-136 L) reactor was charged (E)-3-(3-phenylsulfamoyl-phenyl)-acrylic acid (6) (3.37 kg; 303.34 g/mol; 11.1 mol) and a pre-mixed solution of 1 ,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in isopropyl acetate (IPAc) (27 g in 30 L; 152.24 g/mol; 0.18 mol). The slurry was stirred and thionyl chloride (SOCI2) (960 mL; density ~1.631 g/mL; 118.97 g/mol; -13 mol) was added to the reaction mixture and the batch was stirred at 20-300C overnight. After 18.5 hours, the batch was sampled and deemed to be complete with no compound (6) being detected by HPLC. The resulting solution was transferred to a 100 L Schott reactor for temporary storage while the
30 gallon (-136 L) reactor was rinsed with isopropyl acetate (IPAc) and water. Deionized water (28.9 L) was then added to the 30 gallon (-136 L) reactor followed by 50% (w/w) hydroxylamine (6.57 L; -1.078 g/mL; 33.03 g/mol; -214 mol) and another charge of deionized water (1.66 L) to rinse the lines free of hydroxylamine to make a 10% (w/w) hydroxylamine solution. Tetrahydrofuran (THF) (6.64 L) was then charged to the
30 gallon (-136 L) reactor and the mixture was stirred and cooled to 0-100C. The acid chloride solution (from the 100 L Schott reactor) was then slowly charged into the hydroxylamine solution over 1 hour maintaining a batch temperature of 0-10°C during the addition. The batch was then allowed to warm to 20-300C. The aqueous layer was separated and discarded. The organic layer was then reduced in volume under vacuum while maintaining a batch temperature of less than 300C. The intention was to distill out 10-13 L of solvent, but this level was overshot. A larger volume of isopropyl acetate (IPAc) (16.6 L) was added and about 6 L of solvent was distilled out. The batch had precipitated and heptanes (24.9 L) were added and the batch was held at 20-30°C overnight. The batch was filtered and the residue was washed with heptanes (6.64 L). The filtrate was discarded and the residue was dried at 45-55°C under vacuum with a slight nitrogen bleed over the weekend. The title compound (PXD101) was obtained as a light orange solid (3.11 kg, 89% yield with respect to compound (6)) with a purity of 99.25% (AUC) by HPLC.
The title compound (PXD101) (1.2 kg, 3.77 mol) was dissolved in 8 volumes of 1:1 (EtOH/water) at 600C. Sodium bicarbonate (15.8 g, 5 mol%) was added to the solution. Water (HPLC grade) was then added at a rate of 65 mL/min while keeping the internal temperature >57°C. After water (6.6 L) had been added, crystals started to form and the water addition was stopped. The reaction mixture was then cooled at a rate of 10°C/90 min to a temperature of 0-10cC and then stirred at ambient temperature overnight. The crystals were then filtered and collected. The filter cake was washed by slurrying in water (2 x 1.2 L) and then dried in an oven at 45°C for 60 hours with a slight nitrogen bleed. 1.048 kg (87% recovery) of a light orange solid was recovered. Microscopy and XRPD data showed a conglomerate of irregularly shaped birefringant crystalline particles. The compound was found to contain 0.02% water.
As discussed above: the yield of compound (5) with respect to compound (1) was 69%. the yield of compound (6) with respect to compound (5) was 84%. the yield of PXD101 with respect to compound (6) was 89%.
……………….
FORMULATION
Formulation Studies
These studies demonstrate a substantial enhancement of HDACi solubility (on the order of a 500-fold increase for PXD-101) using one or more of: cyclodextrin, arginine, and meglumine. The resulting compositions are stable and can be diluted to the desired target concentration without the risk of precipitation. Furthermore, the compositions have a pH that, while higher than ideal, is acceptable for use.
UV Absorbance
The ultraviolet (UV absorbance E\ value for PXD-101 was determined by plotting a calibration curve of PXD-101 concentration in 50:50 methanol/water at the λmax for the material, 269 nm. Using this method, the E1i value was determined as 715.7.
Methanol/water was selected as the subsequent diluting medium for solubility studies rather than neat methanol (or other organic solvent) to reduce the risk of precipitation of the cyclodextrin.
Solubility in Demineralised Water
The solubility of PXD-101 was determined to be 0.14 mg/mL for demineralised water. Solubility Enhancement with Cvclodextrins
Saturated samples of PXD-101 were prepared in aqueous solutions of two natural cyclodextrins (α-CD and γ-CD) and hydroxypropyl derivatives of the α, β and Y cyclodextrins (HP-α-CD, HP-β-CD and HP-γ-CD). All experiments were completed with cyclodextrin concentrations of 250 mg/mL, except for α-CD, where the solubility of the cyclodextrin was not sufficient to achieve this concentration. The data are summarised in the following table. HP-β-CD offers the best solubility enhancement for PXD-101.
Phase Solubility Determination of HP-β-CD
The phase solubility diagram for HP-β-CD was prepared for concentrations of cyclodextrin between 50 and 500 mg/mL (5-50% w/v). The calculated saturated solubilities of the complexed HDACi were plotted against the concentration of cyclodextrin. See Figure 1.
………………………..
- Plumb, Jane A.; Finn, Paul W.; Williams, Robert J.; Bandara, Morwenna J.; Romero, M. Rosario; Watkins, Claire J.; La Thangue, Nicholas B.; Brown, Robert (2003). “Pharmacodynamic Response and Inhibition of Growth of Human Tumor Xenografts by the Novel Histone Deacetylase Inhibitor PXD101″. Molecular Cancer Therapeutics 2 (8): 721–728. PMID 12939461.
- “CuraGen Corporation (CRGN) and TopoTarget A/S Announce Presentation of Belinostat Clinical Trial Results at AACR-NCI-EORTC International Conference”. October 2007.
- Final Results of a Phase II Trial of Belinostat (PXD101) in Patients with Recurrent or Refractory Peripheral or Cutaneous T-Cell Lymphoma, December 2009
- “Spectrum adds to cancer pipeline with $350M deal.”. February 2010.
- Helvetica Chimica Acta, 2005 , vol. 88, 7 PG. 1630 – 1657, MP 172
- WO2009/40517 A2, ….
- WO2006/120456 A1, …..
- Synthetic Communications, 2010 , vol. 40, 17 PG. 2520 – 2524, MP 172
- Journal of Medicinal Chemistry, 2011 , vol. 54, 13 PG. 4694 – 4720, NMR IN SUP INFO
| US2008274120 | 11-7-2008 | Histone Deacetylase (Hdac) Inhibitors (Pxd101) for the Treatment of Cancer Alone or in Combination With Chemotherapeutic Agent |
| US2008227845 | 9-19-2008 | CYCLOOXYGENASE-2 INHIBITOR/HISTONE DEACETYLASE INHIBITOR COMBINATION |
| US2008213399 | 9-5-2008 | Combination Therapies Using Hdac Inhibitors |
| US2008194690 | 8-15-2008 | Pharmaceutical Formulations Of Hdac Inhibitors |
| US7407988 | 8-6-2008 | Carbamic acid compounds comprising a sulfonamide linkage as HDAC inhibitors |
| US7402603 | 7-23-2008 | Cyclooxygenase-2 inhibitor/histone deacetylase inhibitor combination |
| US7183298 | 2-28-2007 | Carbamic acid compounds comprising a sulfonamide linkage as HDAC inhibitors |
| US2005107445 | 5-20-2005 | Carbamic acid compounds comprising a sulfonamide linkage as HDAC inhibitors |
| US6888027 | 5-4-2005 | Carbamic acid compounds comprising a sulfonamide linkage as hdac inhibitors |
| WO2002030879A2 | Sep 27, 2001 | Apr 18, 2002 | Prolifix Ltd | Carbamic acid compounds comprising asulfonamide linkage as hdac inhibitors |
| US7973181 | 7-6-2011 | HYDROXAMIC ACID DERIVATIVES AS INHIBITORS OF HDAC ENZYMATIC ACTIVITY |
| US7928081 | 4-20-2011 | Combined Use of Prame Inhibitors and Hdac Inhibitors |
| US2011077305 | 3-32-2011 | 5-LIPOXYGENASE INHIBITORS |
| US2011003777 | 1-7-2011 | Methods of Treatment Employing Prolonged Continuous Infusion of Belinostat |
| US2010286279 | 11-12-2010 | Methods of Synthesis of Certain Hydroxamic Acid Compounds |
| US2010190694 | 7-30-2010 | Methods for identifying patients who will respond well to cancer treatment |
| US2010010010 | 1-15-2010 | HDAC INHIBITORS |
| US2009312311 | 12-18-2009 | COMBINATION OF ORGANIC COMPOUNDS |
| US2009192211 | 7-31-2009 | CYCLOOXYGENASE-2 INHIBITOR/HISTONE DEACETYLASE INHIBITOR COMBINATION |
| US7557140 | 7-8-2009 | CARBAMIC ACID COMPOUNDS COMPRISING A SULFONAMIDE LINKAGE AS HDAC INHIBITORS |
| WO1998038859A1 * | Mar 4, 1998 | Sep 11, 1998 | Thomas E Barta | Sulfonyl divalent aryl or heteroaryl hydroxamic acid compounds |
| WO1999024399A1 * | Nov 12, 1998 | May 20, 1999 | Darwin Discovery Ltd | Hydroxamic and carboxylic acid derivatives having mmp and tnf inhibitory activity |
| WO2000056704A1 * | Mar 22, 2000 | Sep 28, 2000 | Duncan Batty | Hydroxamic and carboxylic acid derivatives |
| WO2000069819A1 * | May 12, 2000 | Nov 23, 2000 | Thomas E Barta | Hydroxamic acid derivatives as matrix metalloprotease inhibitors |
| WO2001038322A1 * | Nov 22, 2000 | May 31, 2001 | Methylgene Inc | Inhibitors of histone deacetylase |
| EP0570594A1 * | Dec 7, 1992 | Nov 24, 1993 | SHIONOGI & CO., LTD. | Hydroxamic acid derivative based on aromatic sulfonamide |
| EP0931788A2 * | Dec 16, 1998 | Jul 28, 1999 | Pfizer Inc. | Metalloprotease inhibitors |
| GB2312674A * | Title not available |
| WO2002030879A2 | Sep 27, 2001 | Apr 18, 2002 | Prolifix Ltd | Carbamic acid compounds comprising a sulfonamide linkage as hdac inhibitors |
| WO2005063806A1 | Dec 30, 2003 | Jul 14, 2005 | Council Scient Ind Res | Arginine hydrochloride enhances chaperone-like activity of alpha crystallin |
| US4642316 | May 20, 1985 | Feb 10, 1987 | Warner-Lambert Company | Parenteral phenytoin preparations |
| WO2008090585A2 * | Jan 25, 2008 | Jul 31, 2008 | Univ Roma | Soluble forms of inclusion complexes of histone deacetylase inhibitors and cyclodextrins, their preparation processes and uses in the pharmaceutical field |
| WO2009109861A1 * | Mar 6, 2009 | Sep 11, 2009 | Topotarget A/S | Methods of treatment employing prolonged continuous infusion of belinostat |
| WO2010048332A2 * | Oct 21, 2009 | Apr 29, 2010 | Acucela, Inc. | Compounds for treating ophthalmic diseases and disorders |
| WO2011064663A1 | Nov 24, 2010 | Jun 3, 2011 | Festuccia, Claudio | Combination treatment employing belinostat and bicalutamide |
| US20110003777 * | Mar 6, 2009 | Jan 6, 2011 | Topotarget A/S | Methods of Treatment Employing Prolonged Continuous Infusion of Belinostat |
………………………..
SPECTRUM
Tiny Biotech With Three Cancer Drugs Is More Alluring Takeover Bet Now
Forbes
The drug is one of Spectrum’s two drugs undergoing phase 3 clinical trials. Allergan paid Spectrum $41.5 million and will make additional payments of up to $304 million based on achieving certain milestones. So far, Raj Shrotriya, Spectrum’s chairman, …
Forbes
The drug is one of Spectrum’s two drugs undergoing phase 3 clinical trials. Allergan paid Spectrum $41.5 million and will make additional payments of up to $304 million based on achieving certain milestones. So far, Raj Shrotriya, Spectrum’s chairman, …
……………………………..
Copenhagen, December 10, 2013
Topotarget announces the submission of a New Drug Application (NDA) for belinostat for the treatment of relapsed or refractory (R/R) peripheral T-cell lymphoma (PTCL) to the US Food and Drug Administration (FDA). The NDA has been filed for Accelerated Approval with a request for Priority Review. Response from the FDA regarding acceptance to file is expected within 60 days from the FDA receipt date.
read all this here
…………………….
..............................................................................
3 VORINOSTAT

Vorinostat
Zolinza, SAHA, suberoylanilide hydroxamic acid, Suberanilohydroxamic acid, N-hydroxy-N'-phenyloctanediamide
US patent 5369108, PDT PATENT
For the treatment of cutaneous manifestations in patients with cutaneous T-cell lymphoma who have progressive, persistent or recurrent disease on or following two systemic therapies. Inhibits histone deacetylase I & 3.
- CCRIS 8456
- HSDB 7930
- M344
- N-Hydroxy-N'-phenyloctanediamide
- SAHA
- SAHA cpd
- Suberanilohydroxamic acid
- suberoylanilide hydroxamic acid
- UNII-58IFB293JI
| N-hydroxy-N'-phenyl-octanediamide | |
|---|---|
| Trade names | Zolinza, 100 MG, CAPSULE, ORAL |
| ZOLINZA (VORINOSTAT) [Merck Sharp & Dohme Corp.] | |
| MedlinePlus | a607050 |
| Licence data | US FDA:link |
| LAUNCHED 2006 MERCKhttp://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021991s002lbl.pdf | |
| Legal status | ℞-only (US) |
| Routes | Oral |
| Pharmacokinetic data | |
| Protein binding | 71% |
| Metabolism | Hepatic glucuronidation andoxidation CYP system not involved |
| Half-life | 2 hours |
| Excretion | Renal (negligible) |
| Identifiers | |
| CAS number | 149647-78-9 |
| ATC code | L01XX38 |
| Chemical data | |
| Formula | C14H20N2O3 |
| Mol. mass | 264.32 g/mol |
CLINICAL TRIALS..http://clinicaltrials.gov/search/intervention=Vorinostat
Vorinostat (rINN) also known as suberanilohydroxamic acid (suberoyl+anilide+hydroxamic acid abbreviated as SAHA) is a member of a larger class of compounds that inhibit histone deacetylases (HDAC). Histone deacetylase inhibitors (HDI) have a broad spectrum of epigenetic activities.
Vorinostat is marketed under the name Zolinza for the treatment of cutaneous T cell lymphoma (CTCL) when the disease persists, gets worse, or comes back during or after treatment with other medicines.[1] The compound was developed by Columbia University chemist, Ronald Breslow.
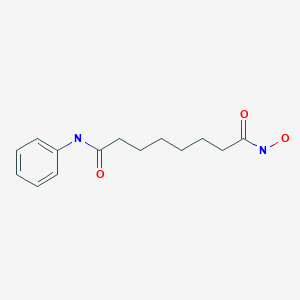 VORINOSTAT
VORINOSTAT
Vorinostat was the first histone deacetylase inhibitor[2] approved by the U.S. Food and Drug Administration (FDA) for the treatment of CTCL on October 6, 2006. It is manufactured by Patheon, Inc., in Mississauga, Ontario, Canada, for Merck & Co., Inc., White House Station, New Jersey.[3]
ZOLINZA contains vorinostat, which is described chemically as N-hydroxy-N'-phenyloctanediamide. The empirical formula is C14H20N2O3. The molecular weight is 264.32 and the structural formula is:
 |
Vorinostat is a white to light orange powder. It is very slightly soluble in water, slightly soluble in ethanol, isopropanol and acetone, freely soluble in dimethyl sulfoxide and insoluble in methylene chloride. It has no chiral centers and is non-hygroscopic. The differential scanning calorimetry ranged from 161.7 (endotherm) to 163.9°C. The pH of saturated water solutions of vorinostat drug substance was 6.6. The pKa of vorinostat was determined to be 9.2.
Each 100 mg ZOLINZA capsule for oral administration contains 100 mg vorinostat and the following inactive ingredients: microcrystalline cellulose, sodium croscarmellose and magnesium stearate. The capsule shell excipients are titanium dioxide, gelatin and sodium lauryl sulfate.
Vorinostat has been shown to bind to the active site of histone deacetylases and act as a chelator for Zinc ions also found in the active site of histone deacetylases [4] Vorinostat's inhibition of histone deacetylases results in the accumulation of acetylated histones and acetylated proteins, including transcription factors crucial for the expression of genes needed to induce cell differentiation. [4]
SAHA inhibits class I and class II HDACs at nanomolar concentrations and arrests cell growth in a wide variety of transformed cells in culture at 2.5-5.0 µM. This compound efficiently suppressed MES-SA cell growth at a low dosage (3 µM) already after 24 hours treatment. Decrease of cell survival was even more pronounced after prolonged treatment and reached 9% and 2% after 48 and 72 hours of treatment, respectively. Colony forming capability of MES-SA cells treated with 3 µM vorinostat for 24 and 48 hours was significantly diminished and blocked after 72 hours.

SAHA inhibits class I and class II HDACs at nanomolar concentrations and arrests cell growth in a wide variety of transformed cells in culture at 2.5-5.0 µM. This compound efficiently suppressed MES-SA cell growth at a low dosage (3 µM) already after 24 hours treatment. Decrease of cell survival was even more pronounced after prolonged treatment and reached 9% and 2% after 48 and 72 hours of treatment, respectively. Colony forming capability of MES-SA cells treated with 3 µM vorinostat for 24 and 48 hours was significantly diminished and blocked after 72 hours.
Vorinostat has also been used to treat Sézary syndrome, another type of lymphoma closely related to CTCL.[5]
A recent study suggested that vorinostat also possesses some activity against recurrent glioblastoma multiforme, resulting in a median overall survival of 5.7 months (compared to 4 - 4.4 months in earlier studies).[6] Further brain tumor trials are planned in which vorinostat will be combined with other drugs.
Including vorinostat in treatment of advanced non-small-cell lung cancer (NSCLC) showed improved response rates and increased median progression free survival and overall survival (although the survival improvements were not significant at the P=0.05 level).[7]
It has given encouraging results in a phase II trial for myelodysplastic syndromes in combination with Idarubicin and Cytarabine.[8]
Vorinostat is an interesting target for scientists interested in eradicating HIV from infected persons.[9] Vorinostat was recently shown to have both in vitro and in vivo effects against latently HIV infected T-cells.[10][11]
Vorinostat, represented by structural formula (I) and chemically named as N-hydroxy-N'- phenyl-octanediamide or suberoylanilide hydroxamic acid (SAElA), is a member of a larger class of compounds that inhibit histone deacetylases (HDAC). Histone deacetylase inhibitors (HDI) have a broad spectrum of epigenetic activities and vorinostat is marketed, under the brand name Zolinza®, for the treatment of a type of skin cancer called cutaneous T-cell lymphoma (CTCL). Vorinostat is approved to be used when the disease persists, gets worse, or comes back during or after treatment with other medicines. Vorinostat has also been used to treat Sέzary's disease and, in addition, possesses some activity against recurrent glioblastoma multiforme.
Vorinostat was first described in US patent 5369108, wherein four different synthetic routes for the preparation of vorinostat are disclosed (Schemes 1 to 4).
The single step process illustrated in Scheme 1 involves coupling of the diacid chloride of suberic acid with aniline and hydiOxylamine hydrochloride. However, the yield of this reaction is only 15-30%.
Scheme 1
The multistep process illustrated in Scheme 2 begins with the monomethyl ester of suberic acid, which undergoes conversion to the corresponding acid chloride. Further coupling with aniline gives the methyl ester of suberanilic acid. Hydrolysis of the ester and further coupling with benzyl protected hydroxylamine gives benzyl protected vorinostat which on deprotection gives vorinostat.
HO. (CH2J6 OMe . ,OOMM e
O O
Scheme 2
In addition to the disadvantage of being a five-step process with overall yields reported as 35-65%, this process suffers from further disadvantages such as the use of the expensive monomethyl ester of suberic acid.
Scheme 3
The two step process illustrated in Scheme 3 involves coupling of the diacid chloride of suberic acid with aniline and O-benzyl hydroxylamine and then deprotection. However, the overall yield of this reaction is only 20-35%.
Scheme 4
The process illustrated in Scheme 4 is similar to that illustrated in Scheme 3, with the exception that O-trimethylsilyl hydroxylamine was used instead of O-benzyl hydroxylamine. The overall yield of this reaction is reported as 20-33%.
Another process for the preparation of vorinostat has been reported in J. Med. Chem.,
1995, vol. 38(8), pages 1411-1413. The reported process, illustrated in Scheme 5, begins with the conversion of suberic acid to suberanilic acid by a high temperature melt reaction.
Suberanilic acid is further converted to the corresponding methyl ester using Dowex resin and the methyl ester of suberanilic acid thus formed is converted to vorinostat by treatment with hydroxylamine hydrochloride. However, this process employs high temperatures (1900C) in the preparation of vorinostat which adds to the inefficiency and high processing costs on commercial scale. The high temperatures also increase the likelihood of impurities being formed during manufacture and safety concerns. The overall yield reported was a poor 35%.
MeOH, Dowex, 22 hours
Scheme 5
Another process for the preparation of vorinostat has been reported in OPPI Briefs, 2001, vol. 33(4), pages 391-394. The reported process, illustrated in Scheme 6, involves conversion of suberic acid to suberic anhydride, which on treatment with aniline gives suberanilic acid. Coupling of this suberanilic acid with ethyl chloroformate gives a mixed anhydride which upon treatment with hydroxylamine gives vorinostat in an overall yield of 58%. In the first step, there is competition between the formation of suberic anhydride and the linear anhydride and consequently isolation of pure suberic anhydride from the reaction mixture is very difficult. This process step is also hindered by the formation of process impurities and competitive reactions. In the second step, there is formation of dianilide by reaction of two moles of aniline with the linear anhydride. In the third step, suberanilic acid is an inconvenient by-product as the suberanilic acid is converted to a mixed anhydride with ethyl chloroformate, which is highly unstable and is converted back into suberanilic acid. Consequently, it is very difficult to obtain pure vorinostat from the reaction mixture. Although the reported yield was claimed to be 58%, when repeated a yield of only 38% was obtained.
Scheme 6
A further process for the preparation of vorinostat has been reported in J. Med. Chem., 2005, vol. 48(15), pages 5047-5051. The reported process, illustrated in Scheme 7, involves conversion of monomethyl suberate to monomethyl suberanilic acid, followed by coupling with hydroxylamine hydrochloride to afford vorinostat in an overall yield of 79%. However, the process uses the expensive monomethyl ester of suberic acid as starting material.
HOBt, DCC, DMF, RT, 4 hours
Processes for the preparation of vorinostat, and its form 1 crystalline polymorph, have been disclosed in patent applications US 2004/0122101 and WO 2006/127319. However, the disclosed processes, comprising the preparation of vorinostat from suberic acid, are a cumbersome three step process comprising the sequential steps of amidation of suberic acid with aniline, esterification of the mono-amide product with methanol, and finally reaction with hydroxylamine hydrochloride and sodium methoxide to afford vorinostat. This process is not very convenient as it involves elevated temperatures, lengthy reaction times and has a low overall yield of around 23%. In addition, the intermediate products and final product are not very pure and require exhaustive purification steps.
.........................
A preferred embodiment of the first aspect of the present invention is illustrated in Scheme
suberic acid subefanilic acid NH2OHHCl, CDI
suberoylanilide hydroxamic acid (T)
Scheme 8
Optionally, an activating agent can be used in step (a) and/ or step (b) to afford products with high yields and purity. Preferably, the activating agent is selected from cyanuric chloride, cyanuric fluoride, catecholborane, or a mixture thereof. The activating agent is preferably used in combination with the coupling agent. A preferred embodiment of the process according to the first aspect of the present invention comprises the following steps:
(i) taking a mixture of THF, CDI and DCC;
(ii) adding suberic acid; (iii) adding aniline in THF to the solution from step (ii);
(iv) stirring at 25-30°C;
(v) filtering off the solid dicyclohexyl urea formed in the reaction;
(vi) concentrating the filtrate in vacuo;
(vii) adding a solution of KOH in water; (vϋi) filtering off the solid by-product;
(ix) heating the filtrate;
(x) adding aq. HCl;
(xi) isolating suberanilic acid;
(xii) mixing the suberanilic acid and CDI in DMF; (xiii) adding hydroxylamine hydrochloride as solid to the mixture from step (xii);
(xiv) isolating vorinostat from the mixture obtained in step (xiii);
(xv) adding acetonitrile and aq. ammonia to the vorinostat from step (xiv);
(xvi) heating the mixture;
(xvii) cooling the mixture to 20-27°C; and (xvϋi) isolating pure vorinostat from the mixture obtained in step (xvii).
Preferably, by utilising the same organic solvent in steps (a) and (b), pure vorinostat can be obtained without isolation of any synthetic intermediate^).
A preferred embodiment of the second aspect of the present invention is illustrated in Scheme 9.
suberic acid N-hydtoxy-7-carboxy-heptanamide
Example 1
Stage 1 : Conversion of suberic acid to suberanilic acid
A mixture of CDI (0.5eq) and DCC (0.8eq) in THF (15 vol) was stirred for 1 hour at 25- 3O0C. Suberic acid (leq) and aniline (leq) in THF (1 vol) was added and the mixture stirred for a further 16-20 hours. The solid by-product was removed by filtration and the filtrate was concentrated in vacuo at 5O0C. The solid residue obtained was treated with a solution of KOH (2eq) in water (10 vol) and stirred for 30 minutes at 25-300C and any solid byproduct formed was removed by filtration. The filtrate obtained was heated at 6O0C for 3-4 hours and cooled to 200C before addition of an aqueous solution of HCl (17.5%, 3 vol). The mixture was stirred for 30 minutes and the solid filtered, washed with water (2x5 vol) and dried under vacuum at 60-650C. Molar Yield = 60-65% Purity by HPLC = 99.5%
Stage 2: Conversion of suberanilic acid to crude vorinostat The suberanilic acid (leq) obtained in stage 1 was dissolved in DMF (5 vol) and CDI (2eq) was added at 25-3O0C and maintained for 30 minutes under stirring. Hydroxylamine hydrochloride (4eq) was added and stirring continued for 30 minutes. Water (25 vol) was then added and the mixture stirred for 2 hours. The precipitated solid was filtered, washed with water (2x5 vol) and dried under vacuum at 500C. Molar Yield = 70-75% Purity by HPLC = 99% Stage 3: Purification of crude vorinostat
Aqueous ammonia (2.5 vol) was added to the crude vorinostat (leq) in acetonitrile (15 vol) at 25-30°C. The mixture was then maintained at 55-60°C for 1 hour before being cooled to 20-25°C and being stirred for a further hour. The resulting solid was filtered, washed with acetonitrile (2x0.5 vol) and dried under vacuum at 45-5O0C for 5 hours. Molar Yield = 55-60% Purity by HPLC > 99.8%
Example 2
Stage 1 : Conversion of suberic acid to crude vorinostat
A mixture of CDI (0.5eq) and DCC (0.8eq) in THF (15 vol) was stirred for 1 hour at 25- 30°C. Suberic acid (leq) and hydroxylamine (leq) in THF (1 vol) was added and the mixture stirred for a further 1 hour. Then CDI (0.5eq), DCC (0.8eq) and aniline (leq) were added to the mixture and the mixture was stirred for a further 16-20 hours. The solid byproduct was removed by filtration and the filtrate was concentrated in vacuo at 50°C to obtain crude vorinostat. Molar Yield = 55-60% Purity by HPLC > 95.8%
Stage 2: Purification of crude vorinostat
Aqueous ammonia (2.5 vol) was added to the crude vorinostat (leq) in acetonitrile (15 vol) at 25-3O0C. The mixture was then maintained at 55-600C for 1 hour before being cooled to 20-250C and being stirred for a further hour. The resulting solid was filtered, washed with acetonitrile (2x0.5 vol) and dried under vacuum at 45-500C for 5 hours. Molar Yield = 35-40% Purity by HPLC > 99.8%
...........................................
SYNTHESIS
Scheme V. - -
Vorinostat
Suberic acid (l.Oeq) was dissolved in tetrahydrofuran (15vol) and the clear solution was chilled to 0-5°C. Methyl chloro formate (l.leq) and triethylamine (1.1 eq) were added to the solution at the same temperature and the mixture was stirred for 15 minutes. The triethylamine.HCl salt formed was filtered off, then aniline (leq) was added to the reaction mixture at 0-50C and stirring was continued for 15 minutes. Methyl chloroformate (l.leq) and triethylamine (l.leq) were added to the clear solution and stirring was continued for a further 15 minutes at 0-5°C. This chilled reaction mixture was added to a freshly prepared hydroxylamine solution in methanol (*see below) chilled to 0-5°C and stirred for 15 minutes at 0-5°C. The solvent was removed under vacuum at 40°C and the residue obtained was taken in methylene dichloride and the organic solution was washed with water and dried over anhydrous sodium sulfate. Methylene dichloride was removed under vacuum at 40°C and acetonitrile was added to the residue. This mixture was stirred for 15 minutes before the solid was filtered under vacuum and dried under vacuum at 60°C to afford the product as a white solid. Molar yield = 35-41%; HPLC purity = 99.90%.
VORINOSTAT
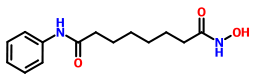
1H-NMR (DMSO-d6): 1.27 (m, 4H, 2 x -CH2-), 1.53 (m, 4H, 2 x -CH2-), 1.94 (t, J = 7.3 Hz, 2H, -CH2-), 2.29 (t, J = 7.4 Hz, 2H, -CH2-), 7.03 (t, J = 7.35 Hz, IH, aromatic para position), 7.27 (t, J = 7.90 Hz, 2H, aromatic meta position), 7.58 (t, J = 7.65 Hz, 2H, aromatic ortho position), 8.66 (s, IH, -OH, D2O exchangeable), 9.85 (s, IH, amide -NH-, D2O exchangeable), 10.33 (s, IH, -NH-OH, D2O exchangeable).
13C-NMR (DMSO-d6): 25.04 (2C, 2 x -CH2-), 28.43 (2C, 2 x -CH2-), 32.24 (1C, -CH2-), 36.34 (1C, -CH2-), 119.01 (2C, Ar-C), 122.96 (1C, Ar-C), 128.68 (2C, Ar-C), 139.24 (1C, Ar- C, =CNH-), 169.23 (1C, -CO-), 171.50 (1C, -CO-).
*Preparation of hydroxylamine solution:
Potassium hydroxide (l.leq) was added to methanol (8vol) and the solution was chilled to 0-5°C. Similarly hydroxylamine hydrochloride (l.leq) was added to methanol (8vol) and chilled to 0-5°C. The chilled amine solution was added to the chilled alkali solution and stirred for 15 minutes at 0-50C. The white potassium chloride salt was filtered off and the filtrate was used as such.
..............................................................
POLYMORPHS
The present invention is directed to a Form I polymorph of SAHA characterized by an X-ray diffraction pattern substantially similar to that set forth in FIG. 13A. SAHA Form I is also characterized by an X-ray diffraction pattern including characteristic peaks at about at about 9.0, 9.4, 17.5, 19.4, 20.0, 24.0, 24.4, 24.8, 25.0, 28.0, and 43.3 degrees 2θ. SAHA Form I is further characterized by an X-ray diffraction pattern including characteristic peaks at about 9.0, 9.4, 17.5, 19.4, 20.0, 24.0, 24.4, 24.8, 25.0, 28.0, 43.3 degrees 20, and lacking at least one peak at about <8.7, 10.0-10.2, 13.4-14.0, 15.0-15.2, 17.5-19.0, 20.1-20.3, 21.1-21.3, 22.0-22.22, 22.7-23.0, 25.0-25.5, 26.0-26.2, and 27.4-27.6 degrees 2θ.
...............................................................
SPECTRAL DATA AND SYNTHESIS
Journal of Medicinal Chemistry, 2011 , vol. 54, 13 pg. 4694 - 4720
for structures see above link
Suberoylanilide hydroxamic acid (26, SAHA, vorinostat).
Suberic acid monomethyl ester (23) (15.09 g, 80.2 mmol) and DMF (0.10 mL) in anhydrous
DCM (300 mL) was added SOCl2 (34.6 mL, 0.481 mol), and the reaction mixture was refluxed for 3
h. The mixture was then concentrated. Toluene (300 mL) was added to the residue and evaporated
to afford crude acid chloride 24. Crude 24 was dissolved in DCM (240 mL), and followed by
addition of aniline (7.3 mL, 80.2 mmol) and Et3N (16.9 mL, 0.120 mol). The reaction mixture was
stirred for 90 min at room temp. The course of reaction was monitored by TLC (30% EtOAc in
hexanes) and LC–MS. DCM was removed, and ethyl acetate (500 mL) was added to dissolve the
residue. The organic layer was washed with aqueous NaHCO3 (500 mL × 2), 1 N HCl (400 mL × 2),
water, dried (Na2SO4), and evaporated to dryness under reduced pressure. The residue was purified
by vacuum liquid chromatography (silica, 20% EtOAc in hexanes) to afford compound 25as white crystalline solids (20.15 g, 96 %). NaOMe in MeOH solution (5.4 M, 106 mL, 0.573 mol) was added to a solution of compound 25 (10.05 g, 38.2 mmol) and NH2OH·HCl (26.54 g, 0.382 mol) in
DCM (300 mL) was added SOCl2 (34.6 mL, 0.481 mol), and the reaction mixture was refluxed for 3
h. The mixture was then concentrated. Toluene (300 mL) was added to the residue and evaporated
to afford crude acid chloride 24. Crude 24 was dissolved in DCM (240 mL), and followed by
addition of aniline (7.3 mL, 80.2 mmol) and Et3N (16.9 mL, 0.120 mol). The reaction mixture was
stirred for 90 min at room temp. The course of reaction was monitored by TLC (30% EtOAc in
hexanes) and LC–MS. DCM was removed, and ethyl acetate (500 mL) was added to dissolve the
residue. The organic layer was washed with aqueous NaHCO3 (500 mL × 2), 1 N HCl (400 mL × 2),
water, dried (Na2SO4), and evaporated to dryness under reduced pressure. The residue was purified
by vacuum liquid chromatography (silica, 20% EtOAc in hexanes) to afford compound 25as white crystalline solids (20.15 g, 96 %). NaOMe in MeOH solution (5.4 M, 106 mL, 0.573 mol) was added to a solution of compound 25 (10.05 g, 38.2 mmol) and NH2OH·HCl (26.54 g, 0.382 mol) in
dry MeOH (375 mL). The reaction mixture was stirred for 40 min at room temp. The reaction was
quenched by adding of 1 N HCl to pH 7–8. MeOH was removed under reduced pressure and water
(1 L) was added to the residue. The precipitated solid was filtered and washed with water (300 mL)
and EtOAc (150 mL) to afford crude 26 which was further purified by recrystallization. MeOH (200
mL) was added to crude 26 (5 g) and warmed to dissolve all solids. The MeOH solution was filtered,
quenched by adding of 1 N HCl to pH 7–8. MeOH was removed under reduced pressure and water
(1 L) was added to the residue. The precipitated solid was filtered and washed with water (300 mL)
and EtOAc (150 mL) to afford crude 26 which was further purified by recrystallization. MeOH (200
mL) was added to crude 26 (5 g) and warmed to dissolve all solids. The MeOH solution was filtered,
and deionized water (400 mL) was added to the filtrate, the resulting solution was placed at 4 oC
overnight. Crystals obtained were filtered and washed with deionized water (100 mL) to afford pure
26 (vorinostat, SAHA) as off-white crystals. Overall yield: 80–85% from compound 23. Compound
26,
overnight. Crystals obtained were filtered and washed with deionized water (100 mL) to afford pure
26 (vorinostat, SAHA) as off-white crystals. Overall yield: 80–85% from compound 23. Compound
26,
LC–MS m/z 265.1 ([M + H]+).

1H NMR (DMSO-d6) 10.35 (1H, s), 9.86 (1H, s), 8.68 (1H, s),
7.58 (2H, d, J = 7.6 Hz), 7.28 (2H, t, J = 7.5 Hz), 7.02 (1H, t, J = 7.4 Hz), 2.29 (2H, t, J = 7.4 Hz),
1.94 (2H, t, J = 7.4 Hz), 1.57 (2H, m), 1.49 (2H, m), 1.33 - 1.20 (2H, m); 13C NMR (DMSO-d6)
171.2, 169.1, 139.3, 128.6, 122.9, 119.0, 36.3, 32.2, 28.4, 28.3, 25.0. Anal. (C10H20N2O3) C, H, N.
7.58 (2H, d, J = 7.6 Hz), 7.28 (2H, t, J = 7.5 Hz), 7.02 (1H, t, J = 7.4 Hz), 2.29 (2H, t, J = 7.4 Hz),
1.94 (2H, t, J = 7.4 Hz), 1.57 (2H, m), 1.49 (2H, m), 1.33 - 1.20 (2H, m); 13C NMR (DMSO-d6)
171.2, 169.1, 139.3, 128.6, 122.9, 119.0, 36.3, 32.2, 28.4, 28.3, 25.0. Anal. (C10H20N2O3) C, H, N.
...................................................................

References
- "ZOLINZA, Merck's Investigational Medicine for Advanced Cutaneous T-Cell Lymphoma (CTCL), To Receive Priority Review from U.S. Food and Drug Administration" (Press release). Merck & Co. June 7, 2006. Retrieved 2006-10-06.
- HDAC Inhibitors Base (vorinostat)
- "FDA Approves New Drug for Skin Cancer, Zolinza" (Press release). Food and Drug Administration. October 6, 2006. Retrieved 2006-10-06.
- Richon, Victoria. "Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor". British Journal of Cancer. Retrieved 3 May 2012.
- Cuneo A, Castoldi. "Mycosis fungoides/Sezary's syndrome". Retrieved 2008-02-15.
- "Vorinostat shows anti-cancer activity in recurrent gliomas" (Press release). Mayo Clinic. June 3, 2007. Retrieved 2007-06-03.
- http://www.rtmagazine.com/reuters_article.asp?id=20091209clin013.html Dec 2009. URL dead Jan 2012
- "Zolinza, Idarubicin, Cytarabine Combination Yields High Response Rates In MDS Patients (ASH 2011)".
- "Study of the Effect of Vorinostat on HIV RNA Expression in the Resting CD4+ T Cells of HIV+ Pts on Stable ART". ClinicalTrials.gov. 2011-03-21.
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM (2009). "Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid.". AIDS Res Hum Retroviruses 25 (2): 207–12. doi:10.1089/aid.2008.0191. PMC 2853863. PMID 19239360.
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J et al. (2009). "Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells.". J Biol Chem 284 (11): 6782–9.doi:10.1074/jbc.M807898200. PMC 2652322. PMID 19136668.
- Vorinostat bound to proteins in the PDB
- J. Med. Chem.,1995, vol. 38(8), pages 1411-1413.
- A new simple and high-yield synthesis of suberoylanilide hydroxamic acid and its inhibitory effect alone or in combination with retinoids on proliferation of human prostate cancer cells
J Med Chem 2005, 48(15): 5047 - A new facile and expeditious synthesis of N-hydroxy-N'-phenyloctanediamide, a potent inducer of terminal cytodifferentiation
Org Prep Proced Int 2001, 33(4): 391 - US patent 5369108, PDT PATENT
- WO2007/22408.........
- WO 1993007148
- CN 102344392
| United States | 7456219 | APPROVAL 2006-11-14 | EXPIRY 2026-11-14 |
| United States | 6087367 | 1994-10-04 | 2011-10-04 |
| Canada | 2120619 | 2006-11-21 | 2012-10-05 |
| Patent | Patent Expiry | pat use code |
|---|---|---|
| 7399787 | Feb 9, 2025 | U-892 |
| 7456219 | Mar 11, 2027 | |
| 7652069 | Mar 4, 2023 | |
| 7732490 | Mar 4, 2023 | U-892 |
| 7851509 | Feb 21, 2024 | U-892 |
| 8067472 | Mar 4, 2023 | U-892 |
| 8093295 | May 16, 2026 | |
| 8101663 | Mar 4, 2023 | U-892 |
| RE38506 | Nov 29, 2013 |
U 892 =TREATMENT OF CUTANEOUS MANIFESTATIONS IN PATIENTS WTIH CUTANEOUS T-CELL LYMPHOMA (CTCL)
| Exclusivity Code | Exclusivity_Date |
|---|---|
| ODE | Oct 6, 2013 |
| WO2009098515A1 * | Feb 6, 2009 | Aug 13, 2009 | Generics Uk Ltd | Novel process for the preparation of vorinostat |
Marks, P.A., Breslow, R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotech 25(1) 84-90 (2007). DOI: 10.1038/nbt1272
Takashi Kumagai, et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. International Journal of Cancer. 2007 Aug 1;121(3):656-65. DOI: 10.1002/ijc.22558
Hrzenjak A, et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol Cancer. 2010 Mar 4;9:49. DOI: 10.1186/1476-4598-9-49
Takashi Kumagai, et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. International Journal of Cancer. 2007 Aug 1;121(3):656-65. DOI: 10.1002/ijc.22558
Hrzenjak A, et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol Cancer. 2010 Mar 4;9:49. DOI: 10.1186/1476-4598-9-49
...................................................................................................
| US7148257 | Aug 26, 2003 | Dec 12, 2006 | Merck Hdac Research, Llc | Methods of treating mesothelioma with suberoylanilide hydroxamic acid |
| US7375137 | Mar 28, 2006 | May 20, 2008 | Merck Hdac Research, Llc | Methods of treating cancer with HDAC inhibitors |
| US7399787 | Jul 9, 2003 | Jul 15, 2008 | Merck Hdac Research, Llc | Methods of treating cancer with HDAC inhibitors |
| US7456219 | Jun 19, 2003 | Nov 25, 2008 | Merck Hdac Research, Llc | Polymorphs of suberoylanilide hydroxamic acid |
| US7652069 | Oct 30, 2007 | Jan 26, 2010 | Merck Hdac Research, Llc | Polymorphs of suberoylanilide hydroxamic acid |
| US7732490 | Sep 11, 2007 | Jun 8, 2010 | Merck Hdac Research, Llc | Methods of treating cancer |
| US7847122 | Mar 18, 2008 | Dec 7, 2010 | Merck Hdac Research, Llc | Polymorphs of suberoylanilide hydroxamic acid |
| US7851509 | Mar 18, 2008 | Dec 14, 2010 | Merck Hdac Research, Llc | Polymorphs of suberoylanilide hydroxamic acid |
| US7879865 | Nov 18, 2005 | Feb 1, 2011 | Sloan-Kettering Institute For Cancer Research | Treatment of cancer of the brain using histone deacetylase inhibitors |
| US7998957 | Feb 6, 2008 | Aug 16, 2011 | Lixte Biotechnology, Inc. | Oxabicycloheptanes and oxabicylcoheptenes, their preparation and use |
| US8058268 | Jul 29, 2009 | Nov 15, 2011 | Lixte Biotechnology, Inc. | Neuroprotective agents for the prevention and treatment of neurodegenerative diseases |
| US8067472 | Apr 23, 2010 | Nov 29, 2011 | Merck Hdac Research, Llc | Methods of treating Hodgkin's and non-Hodgkin's lymphoma |
| US8088951 | Nov 30, 2007 | Jan 3, 2012 | Massachusetts Institute Of Technology | Epigenetic mechanisms re-establish access to long-term memory after neuronal loss |
| US8093295 | May 16, 2006 | Jan 10, 2012 | Merck Sharp & Dohme Corp. | Formulations of suberoylanilide hydroxamic acid and methods for producing the same |
| US8101663 | Dec 7, 2009 | Jan 24, 2012 | Merck Hdac Research, Llc | Polymorphs of suberoylanilide hydroxamic acid |
| US8143445 | Oct 1, 2008 | Mar 27, 2012 | Lixte Biotechnology, Inc. | HDAC inhibitors |
| US8227473 | Jul 17, 2009 | Jul 24, 2012 | Lixte Biotechnology, Inc. | Oxabicycloheptanes and oxabicycloheptenes, their preparation and use |
| US8288440 * | Jan 13, 2010 | Oct 16, 2012 | Merck Sharp & Dohme Corp. | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| US8329719 | Aug 1, 2011 | Dec 11, 2012 | Lixte Biotechnology, Inc. | Neuroprotective agents for the prevention and treatment of neurodegenerative diseases |
| US8426444 | Jun 30, 2011 | Apr 23, 2013 | Lixte Biotechnology, Inc. | Oxabicycloheptanes and oxabicycloheptenes, their preparation and use |
| US8450372 * | Jan 13, 2010 | May 28, 2013 | Merck Sharp & Dohme Corp. | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| US8455688 | Mar 21, 2012 | Jun 4, 2013 | Lixte Biotechnology, Inc. | HDAC inhibitors |
| US8541458 | Jun 11, 2012 | Sep 24, 2013 | Lixte Biotechnology, Inc. | Oxabicycloheptanes and oxabicycloheptenes, their preparation and use |
| US8563615 | Nov 1, 2010 | Oct 22, 2013 | Massachusetts Institute Of Technology | Use of CI-994 and dinaline for the treatment of memory/cognition and anxiety disorders |
| US20100112046 * | Jan 13, 2010 | May 6, 2010 | Jeannie Chow Wong | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| US20100113829 * | Jan 13, 2010 | May 6, 2010 | Cote Aaron S | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| US20100119596 * | Jan 13, 2010 | May 13, 2010 | Jeannie Chow Wong | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| US20110263712 * | Oct 14, 2009 | Oct 27, 2011 | Generics (Uk) Limited | Process for the preparation of vorinostat |
| US20110313044 * | Jun 16, 2011 | Dec 22, 2011 | Urquima S.A. | Polymorphs of Suberoylanilide Hydroxamic Acid |
| EP2079304A1 * | Sep 24, 2007 | Jul 22, 2009 | Merck & Co., Inc. | Amine base salts of saha and polymorphs thereof |
| EP2229941A1 * | May 16, 2006 | Sep 22, 2010 | Merck Sharp & Dohme Corp. | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| EP2292221A2 * | May 16, 2006 | Mar 9, 2011 | Merck Sharp & Dohme Corp. | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| WO2006127319A2 * | May 16, 2006 | Nov 30, 2006 | Merck & Co Inc | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| WO2006127321A2 * | May 16, 2006 | Nov 30, 2006 | Merck & Co Inc | Formulations of suberoylanilide hydroxamic acid and methods for producing same |
| WO2008039421A2 * | Sep 24, 2007 | Apr 3, 2008 | Arlene E Mckeown | Pharmaceutical compositions of hdac inhibitors and chelatable metal compounds, and metal-hdac inhibitor chelate complexes |
| WO2008042146A1 * | Sep 24, 2007 | Apr 10, 2008 | Arlene E Mckeown | Amine base salts of saha and polymorphs thereof |
| WO2008097654A1 * | Feb 8, 2008 | Aug 14, 2008 | Nancie M Archin | Methods of using saha for treating hiv infection |
| WO2009020565A1 * | Aug 1, 2008 | Feb 12, 2009 | Lixte Biotechnology Inc | Use of phosphatases to treat neuroblastomas and medulloblastomas |
| WO2010061220A2 * | Nov 25, 2009 | Jun 3, 2010 | Generics [Uk] Limited | Novel processes and pure polymorphs |
.....................................................................
4 MOCETINOSTAT

Mocetinostat
CAS 726169-73-9;
MGCD0103; MGCD-0103; MGCD 0103;
N-(2-AMINOPHENYL)-4-([[4-(PYRIDIN-3-YL)PYRIMIDIN-2-YL]AMINO]METHYL)BENZAMIDE
N-(2-Amino-phenyl)-4-[(4-pyridin-3-pyrimidin-2-ylamino)-methyl]-benzamide
Molecular Formula: C23H20N6O
Molecular Weight: 396.4445
SAN DIEGO, Aug. 11, 2014 /PRNewswire/ -- Mirati Therapeutics, Inc. (NASDAQ: MRTX) today announced that the U.S. FDA has granted Orphan Drug Designation to mocetinostat, a spectrum selective HDAC inhibitor, for diffuse large B-cell lymphoma (DLBCL). In June, mocetinostat was granted Orphan Drug Designation as a treatment for myelodysplastic syndrome (MDS). Orphan drug designation is also being sought for bladder cancer patients with specific genetic alterations.
| Identifiers | |
|---|---|
| CAS number | 726169-73-9 |
| PubChem | 9865515 |
| ChemSpider | 8041206 |
| ChEMBL | CHEMBL272980 |
| Jmol-3D images | Image 1 |
| Properties | |
| Molecular formula | C23H20N6O |
| Molar mass | 396.44 g mol−1 |
Mocetinostat (MGCD0103) is a benzamide histone deacetylase inhibitor undergoing clinical trials for treatment of various cancers including follicular lymphoma, Hodgkin's lymphoma and acute myelogenous leukemia.[1][2][3]
One clinical trial (for refractory follicular lymphoma) was temporarily put on hold due to cardiac problems but resumed recruiting in 2009.[4]
In 2010 favourable results were announced from the phase II trial for Hodgkin's lymphoma.[5]
MGCD0103 has also been used as a research reagent where blockage of members of the HDAC-family of histone deacetylases is required.[6]
Mechanism of action
About Mocetinostat
Mocetinostat is an orally-bioavailable, spectrum-selective HDAC inhibitor. Mocetinostat is enrolling patients in a Phase 2 dose confirmation study in combination with Vidaza as treatment for intermediate and high-risk MDS. Mirati also plans to initiate Phase 2 studies of mocetinostat as a single agent in patients with mutations in histone acetyl transferases in bladder cancer and DLBCL. Initial data from the Phase 2 studies is expected by the end of 2014. In addition to the ongoing Phase 2 clinical trials, mocetinostat has completed 13 clinical trials in more than 400 patients with a variety of hematologic malignancies and solid tumors.
About Mirati Therapeutics
Mirati Therapeutics is a targeted oncology company developing an advanced pipeline of breakthrough medicines for precisely defined patient populations. Mirati's approach combines the three most important factors in oncology drug development - drug candidates with complementary and compelling targets, creative and agile clinical development, and a highly accomplished precision medicine leadership team. The Mirati team is using a proven blueprint for developing targeted oncology medicines to advance and maximize the value of its pipeline of drug candidates, including MGCD265 and MGCD516, which are orally bioavailable, multi-targeted kinase inhibitors with distinct target profiles, and mocetinostat, an orally bioavailable, spectrum-selective histone deacetylase inhibitor. More information is available at www.mirati.com.
In eukaryotic cells, nuclear DNA associates with histones to form a compact complex called chromatin. The histones constitute a family of basic proteins which are generally highly conserved across eukaryotic species. The core histones, termed H2A, H2B, H3, and H4, associate to form a protein core. DNA winds around this protein core, with the basic amino acids of the histones interacting with the negatively charged phosphate groups of the DNA. Approximately 146 base pairs of DNA wrap around a histone core to make up a nucleosome particle, the repeating structural motif of chromatin.
Csordas, Biochem. J., 286: 23-38 (1990) teaches that histones are subject to posttranslational acetylation of the α,ε-amino groups of N-terminal lysine residues, a reaction that is catalyzed by histone acetyl transferase (HAT1). Acetylation neutralizes the positive charge of the lysine side chain, and is thought to impact chromatin structure. Indeed, Taunton et al., Science, 272: 408-411 (1996), teaches that access of transcription factors to chromatin templates is enhanced by histone hyperacetylation. Taunton et al. further teaches that an enrichment in underacetylated histone H4 has been found in transcriptionally silent regions of the genome.
Histone acetylation is a reversible modification, with deacetylation being catalyzed by a family of enzymes termed histone deacetylases (HDACs). Grozinger et al., Proc. Natl. Acad. Sci. USA, 96: 4868-4873 (1999), teaches that HDACs are divided into two classes, the first represented by yeast Rpd3-like proteins, and the second represented by yeast Hda1-like proteins. Grozinger et al. also teaches that the human HDAC1, HDAC2, and HDAC3 proteins are members of the first class of HDACs, and discloses new proteins, named HDAC4, HDAC5, and HDAC6, which are members of the second class of HDACs. Kao et al., Genes & Dev., 14: 55-66 (2000), discloses HDAC7, a new member of the second class of HDACs. More recently, Hu et al. J. Bio. Chem. 275:15254-13264 (2000) and Van den Wyngaert, FEBS, 478: 77-83 (2000) disclose HDAC8, a new member of the first class of HDACs.
Richon et al., Proc. Natl. Acad. Sci. USA, 95: 3003-3007 (1998), discloses that HDAC activity is inhibited by trichostatin A (TSA), a natural product isolated from Streptomyces hygroscopicus, and by a synthetic compound, suberoylanilide hydroxamic acid (SAHA). Yoshida and Beppu, Exper. Cell Res., 177: 122-131 (1988), teaches that TSA causes arrest of rat fibroblasts at the G1 and G2 phases of the cell cycle, implicating HDAC in cell cycle regulation. Indeed, Finnin et al., Nature, 401: 188-193 (1999), teaches that TSA and SAHA inhibit cell growth, induce terminal differentiation, and prevent the formation of tumors in mice. Suzuki et al., U.S. Pat. No. 6,174,905, EP 0847992, JP 258863/96, and Japanese Application No. 10138957, disclose benzamide derivatives that induce cell differentiation and inhibit HDAC. Delorme et al., WO 01/38322 and PCT/IB01/00683, disclose additional compounds that serve as HDAC inhibitors.
The molecular cloning of gene sequences encoding proteins with HDAC activity has established the existence of a set of discrete HDAC enzyme isoforms. Some isoforms have been shown to possess specific functions, for example, it has been shown that HDAC-6 is involved in modulation of microtubule activity. However, the role of the other individual HDAC enzymes has remained unclear.
These findings suggest that inhibition of HDAC activity represents a novel approach for intervening in cell cycle regulation and that HDAC inhibitors have great therapeutic potential in the treatment of cell proliferative diseases or conditions. To date, few inhibitors of histone deacetylase are known in the art.
....................
Mocetinostat (MGCD-0103)
N-(2-aminophenyl)-4-[[(4-pyridin-3-ylpyrimidin-2-yl)amino]methyl^^
..............................
Example 426 Synthesis of N-(2-Amino-phenyl)-4-[(4-pyridin-3-pyrimidin-2-ylamino)-methyl]-benzamide
Step 1: Synthesis of 4-Guanidinomethyl-benzoic acid methyl ester Intermediate 1
The mixture of 4-Aminomethyl-benzoic acid methyl ester HCl (15.7 g, 77.8 mmol) in DMF (85.6 mL) and DIPEA (29.5 mL, 171.2 mmol) was stirred at rt for 10 min. Pyrazole-1-carboxamidine HCl (12.55 g, 85.6 mmol) was added to the reaction mixture and then stirred at rt for 4 h to give clear solution. The reaction mixture was evaporated to dryness under vacuum. Saturated NaHCO3 solution (35 mL) was added to give nice suspension. The suspension was filtered and the filter cake was washed with cold water. The mother liquid was evaporated to dryness and then filtered. The two solids were combined and re-suspended over distilled H2O (50 ml). The filter cake was then washed with minimum quantities of cold H2O and ether to give 12.32 g white crystalline solid intermediate 1 (77% yield, M+1: 208 on MS).
Step 2: Synthesis of 3-Dimethylamino-1-pyridin-3-yl-propenone Intermediate 2
3-Acetyl-pyridine (30.0 g, 247.6 mmol) and DMF dimethyl acetal (65.8 mL, 495.2 mmol) were mixed together and then heated to reflux for 4 h. The reaction mixture was evaporated to dryness and then 50 mL diethyl ether was added to give brown suspension. The suspension was filtered to give 36.97 g orange color crystalline product (85% yield, M+1: 177 on MS).
Step 3: Synthesis of 4-[(4Pyridin-3-pyrimidin-2-ylamino)-methyl]benzoic acid methyl ester Intermediate 3
Intermediate 1 (0.394 g, 1.9 mmol) and intermediate 2 (0.402 g, 2.3 mmol) and molecular sieves (0.2 g, 4A, powder, >5 micron) were mixed with isopropyl alcohol (3.8 mL). The reaction mixture was heated to reflux for 5 h. MeOH (50 mL) was added and then heated to reflux. The cloudy solution was filtrated over a pad of celite. The mother liquid was evaporated to dryness and the residue was triturated with 3 mL EtOAc. The suspension was filtrated to give 0.317 g white crystalline solid Intermediate 3 (52%, M+1: 321 on MS).
Step 4: Synthesis of N-(2-Amino-phenyl)-4-[(4-pyrymidin-2-ylamino)-methyl]-benzamide
Intermediate 3 (3.68 g, 11.5 mmol) was mixed with THF (23 mL), MeOH (23 mL) and H2O (11.5 mL) at rt. LiOH (1.06 g, 25.3 mmol) was added to reaction mixture. The resulting reaction mixture was warmed up to 40° C. overnight. HCl solution (12.8 mL, 2N) was added to adjust pH=3 when the mixture was cooled down to rt. The mixture was evaporated to dryness and then the solid was washed with minimum quantity of H2O upon filtration. The filter cake was dried over freeze dryer to give 3.44 g acid of the title compound (95%, M+1: 307 on MS).
Acid (3.39 g, 11.1 mmol) of the title compound, BOP (5.679 g, 12.84 mmol) and o-Ph(NH2)2 (2.314 g, 21.4 mmol) were dissolved in the mixture of DMF (107 mL) and Et3N (2.98 mL, 21.4 mmol). The reaction mixture was stirred at rt for 5 h and then evaporated to dryness. The residue was purified by flash column (pure EtOAc to 5% MeOH/EtOAc) and then interested fractions were concentrated. The final product was triturated with EtOAc to give 2.80 g of title product
(66%, MS+1: 397 on MS).
1H NMR (400 MHz, DMSO-D6) δ (ppm): 9.57 (s, 1H), 9.22 (s, 1H), 8.66 (d, J=3.5 Hz, 1H), 8.39 (d, J=5.1 Hz, 2H), 8.00 (t, J=6.5 Hz, 1H), 7.90 (d, J=8.2 Hz, 2H), 7.50 (m, 3H), 7.25 (d, J=5.1 Hz, 1H), 7.12 (d, J=7.4 Hz, 1H), 6.94 (dd, J=7.0, 7.8 Hz, 1H), 6.75 (d, J=8.2 Hz, 1H), 6.57 (dd, J=7.0, 7.8 Hz, 1H), 4.86 (s, 2H), 4.64 (d, J=5.9 Hz, 2H).
References
- "Pharmion Corporation (PHRM) Release: Clinical Data On Oncology HDAC Inhibitor MGCD0103, Presented At The American Society of Clinical Oncology 42nd Annual Meeting" (Press release). Colorado, United States: BioSpace. June 6, 2006.
- Gelmon, K.; Tolcher, A.; Carducci, M.; Reid, G. K.; Li, Z.; Kalita, A.; Callejas, V.; Longstreth, J. et al. (2005). "Phase I trials of the oral histone deacetylase (HDAC) inhibitor MGCD0103 given either daily or 3x weekly for 14 days every 3 weeks in patients (pts) with advanced solid tumors". J. Clin. Oncol. 2005 ASCO Annual Meeting. 23 (16S). 3147.
- MethylGene to Resume Development of its HDAC Inhibitor, MGCD0103 (Mocetinostat), Sept 2009
- "METHYLGENE TO RESUME DEVELOPMENT OF ITS HDAC INHIBITOR, MGCD0103 (MOCETINOSTAT)". 21 Sep 2009.
- "Final Phase 2 Clinical Data for Mocetinostat (MGCD0103) in Relapsed/Refractory Hodgkin Lymphoma Patients". 6 Dec 2010.
- Pfefferli, Catherine; Müller, Fritz; Ja¿wi¿ska, Anna; Wicky, Chantal (2014). "Specific NuRD components are required for fin regeneration in zebrafish". BMC Biol. 12 (30). doi:10.1186/1741-7007-12-30. PMID 24779377.
- MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo
3-20-2009
|
THERAPEUTIC COMBINATIONS AND METHODS FOR CARDIOVASCULAR IMPROVEMENT AND TREATING CARDIOVASCULAR DISEASE
| |
10-3-2008
|
COMBINATION OF ERa+ LIGANDS AND HISTONE DEACETYLASE INHIBITORS FOR THE TREATMENT OF CANCER
| |
12-21-2007
|
Assay for efficacy of histone deacetylase inhibitors
| |
5-25-2005
|
Inhibitors of histone deacetylase
|
2-8-2012
|
HDAC INHIBITORS AND HORMONE TARGETED DRUGS FOR THE TREATMENT OF CANCER
| |
6-3-2011
|
Sequential Administration of Chemotherapeutic Agents for Treatment of Cancer
| |
5-6-2011
|
METHODS FOR TREATING OR PREVENTING COLORECTAL CANCER
| |
1-12-2011
|
Inhibitors of histone deacetylase
| |
1-12-2011
|
Inhibitors of Histone Deacetylase
| |
11-24-2010
|
Inhibitors of histone deacetylase
| |
3-5-2010
|
INTRAOCULAR PRESSURE-LOWERING AGENT COMPRISING COMPOUND HAVING HISTONE DEACETYLASE INHIBITOR EFFECT AS ACTIVE INGREDIENT
| |
6-12-2009
|
Administration of an Inhibitor of HDAC and an mTOR Inhibitor
| |
5-22-2009
|
Combinations of HDAC Inhibitors and Proteasome Inhibitors
| |
5-15-2009
|
Combination Therapy
|
.....................................................
.................................................................
5 EZATIOSTAT
Ezatiostat
168682-53-9 (Ezatiostat); 286942-97-0 (Ezatiostat HCl salt)
gamma-Glu-S-BzCys-PhGly diethyl ester
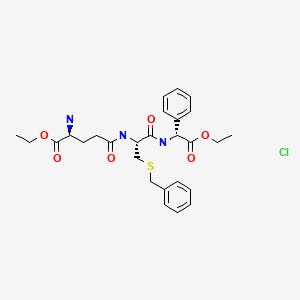 HCl; salt, D08917,
HCl; salt, D08917, Ezatiostat hydrochloride
Target: glutathione S-transferase P1-1 (GSTP1-1) inhibitorPathway: hsa00480 Glutathione metabolism
Activity: Treatment of disorders of bone marrow cellular growth and differentiation
see http://www.ncbi.nlm.nih.gov/pubmed?term=TLK-199&cmd=search
| Telik, Inc. |
TLK199; TLK-199; TLK 199; Brand name: TELINTRA®。ethyl (2R)-[(4S)-4-amino-5-ethoxy-5-oxopentanoyl]-S-benzyl-L-cysteinyl-2- phenylglycinate.
ethyl
(2S)-2-amino-5-[[(2R)-3-benzylsulfanyl-1-[[(1R)-2-ethoxy-2-oxo-1-phenylethyl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoate.
IUPAC/Chemical name:
(S)-ethyl 2-amino-5-(((R)-3-(benzylthio)-1-(((S)-2-ethoxy-2-oxo-1-phenylethyl)amino)-1-oxopropan-2-yl)amino)-5-oxopentanoate
C27H35N3O6SExact Mass: 529.2246
nmr.http://www.medkoo.com/Product-Data/Ezatiostat/ezatiostat-QC-CRB40225web.pdf
Telintra is a small molecule product candidate designed to stimulate the production of blood cells in the bone marrow. Many conditions are characterized by depleted bone marrow, including myelodysplastic syndrome, a form of pre-leukemia in which the bone marrow produces insufficient levels of one or more of the 3 major blood elements (white blood cells, red blood cells and platelets). A reduction in blood cell levels is also a common, toxic effect of many standard chemotherapeutic drugs.
Ezatiostat is a liposomal small-molecule glutathione analog inhibitor of glutathione S-transferase (GST) P1-1 with hematopoiesis-stimulating activity. After intracellular de-esterification, the active form of ezatiostat binds to and inhibits GST P1-1, thereby restoring Jun kinase and MAPK pathway activities and promoting MAPK-mediated cellular proliferation and differentiation pathways. This agent promotes the proliferation and maturation of hematopoietic precursor cells, granulocytes, monocytes, erythrocytes and platelets
Phase II trial myelodysplastic syndrome (MDS): Cancer. 2012 Apr 15;118(8):2138-47.
Phase I trial myelodysplastic syndrome (MDS): J Hematol Oncol. 2012 Apr 30;5:18. doi: 10.1186/1756-8722-5-18; Blood. 2009 Jun 25;113(26):6533-40; J Hematol Oncol. 2009 May 13;2:20.
Ezatiostat hydrochloride is the hydrochloride acid addition salt of
ezatiostat. Ezatiostat, also known as TLK199 or TER 199, is a compound
of the formula:Ezatiostat has been shown to induce the differentiation of HL-60 promyelocyte leukemia cells in vitro, to potentiate the activity of cytotoxic agents both in vitro and in vivo, and to stimulate colony formation of all three lineages of hematopoietic progenitor cells in normal human peripheral blood.
In preclinical testing, ezatiostat has been shown to increase white blood cell production in normal animals, as well as in animals in which white blood cells were depleted by treatment with cisplatin or fluorouracil. Similar effects may provide a new approach to treating myelodysplastic syndrome (MDS).
Many conditions, including MDS, a form of pre-leukemia in which the bone marrow produces insufficient levels of one or more of the three major blood elements (white blood cells, red blood cells, and platelets), are characterized by depleted bone marrow. Myelosuppression, which is characterized by a reduction in blood cell levels and in a reduction of new blood cell generation in the bone marrow, is also a common, toxic effect of many standard chemotherapeutic drugs.
Ezatiostat hydrochloride in a liposomal injectable formulation was studied in a clinical trial for the treatment of MDS, and results from this trial, reported by Raza et al., J Hem. One, 2:20 (published online 13 May 2009), demonstrated that administration of TLK199 was well tolerated and resulted in multi-lineage hematologic improvement.
Ezatiostat hydrochloride in a tablet formulation has been evaluated in a clinical trial for the treatment of MDS, as reported by Raza et al., Blood, 113:6533-6540 (prepublished online 27 April 2009) and a single-patient report by Quddus et al., J Hem. One, 3:16 (published online 23 April 2010), and is currently being evaluated in clinical trials for the treatment of MDS and for severe chronic idiopathic neutropenia.
When used for treating humans, it is important that a crystalline therapeutic agent like ezatiostat hydrochloride retains its polymorphic and chemical stability, solubility, and other physicochemical properties over time and among various manufactured batches of the agent. If the physicochemical properties vary with time and among batches, the administration of a therapeutically effective dose becomes problematic and may lead to toxic side effects or to ineffective therapy, particularly if a given polymorph decomposes prior to use, to a less active, inactive, or toxic compound.
Therefore, it is important to choose a form of the crystalline agent that is stable, is manufactured reproducibly, and has physicochemical properties favorable for its use as a therapeutic agent.
Ezatiostat hydrochloride (USAN) has the molecular weight of 566.1, the trademark of Telintra®, and the CAS registry number of 286942-97-0. Ezatiostat hydrochloride has been evaluated for the treatment of myelodysplastic syndrome (MDS), in a Phase I-IIa study using a liposomal formulation (U.S. Pat. No. 7,029,695), as reported at the 2005 Annual Meeting of the American Society for Hematology (Abstract #2250) and by Raza et al. in Journal of Hematology & Oncology, 2:20 (published online on 13 May 2009); and in a Phase I study using a tablet formulation, as reported at the 2007 Annual Meeting of the American Society for Hematology (Abstract #1454) and by Raza et al. in Blood, 113:6533-6540 (prepublished online on 27 Apr. 2009), and in a single patient case report by Quddus et al. in Journal of Hematology & Oncology, 3:16 (published online on 23 Apr. 2010).
…………………………………………………………………
http://www.google.com/patents/US20110301376
Preparation of Ezatiostat Hydrochloride
In another aspect, this invention provides a process comprising the steps of contacting a compound of formula:
or a salt thereof with a compound of formula:
or a salt thereof and an activating agent under conditions which provide a compound of formula:
In one embodiment, the process further comprises deprotecting the compound of formula:
under conditions which provide a compound of formula:
or a salt thereof. In another embodiment, the compound provided is ezatiostat hydrochloride.
In another aspect, this invention provides a process comprising contacting a compound of formula:
or a salt thereof with an ethylating agent under conditions which provide a compound of formula:
In another embodiment, the process further comprises debenzylating the compound of formula:
under conditions which provide a compound of formula:
or a salt thereof.
In another aspect, this invention provides a process comprising the steps of contacting a compound of formula:
or a salt thereof having a t-butoxycarbonyl group with an activating agent and a compound of formula:
or a salt thereof under conditions which provide a compound of formula:
In another embodiment, the process further comprises deprotecting the tertiarybutyloxycarboyl (Boc) group under conditions to provide a compound of formula:
or a salt thereof.
Certain preferred embodiments of this invention are illustrated in the reaction scheme and described below. In the peptide coupling the amino acid reagents are used generally at a 1:1 molar ratio, and the activating reagent (isobutyl chloroformate) and the base (NMM) are used in slight excess over the amino acid reagents; while in the esterification of the N-BOC-L-glutamic acid γ-benzyl ester the esterifying agent (diethyl sulfate) and base are used in about 1.4-fold excess.
EXAMPLESAs relevant and unless otherwise noted, all operations were
conducted under nitrogen purge and with stirring. Water was osmosis
purified, and solvents were filtered. Unless otherwise stated, all
temperatures are in degrees Celcius (° C.) and the following
abbreviations have the following definitions:Et EthylHCl(g) HCl gasN-BOC
or N-Boc N-tertiarybutyloxycarbonylL LiterKg KilogramNMM
N-methylmorpholineMol Molew/w weight by weightExample 1Preparation of
S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride
(3)Without stirring, 45.1 Kg N-BOC-S-benzyl-L-cysteine (1) was added to a
600 L jacketed glass-lined reactor, followed by 45 L ethyl acetate.
Stirring was started and the temperature was reduced to 13° C. NMM, 15.3
Kg, was added over 50 minutes, and rinsed in with 6 L ethyl acetate,
and stirring stopped. Ethyl acetate, 315 L, was added to an 800 L cooled
jacketed glass-lined reactor, followed by 20.7 Kg isobutyl
chloroformate, rinsed in with 11 L ethyl acetate, and the mixture cooled
to −10° C. The N-BOC-S-benzyl-L-cysteine NMM salt solution was added to
the 800 L reactor over 5 hours, its reactor rinsed with 11 L ethyl
acetate, and the rinse solution added to the 800 L reactor, while
maintaining the temperature at (−10˜−7)° C. D-Phenylglycine ethyl ester
hydrochloride, 31.2 Kg, was added in 8 portions over 50 minutes,
followed by 15.3 Kg NMM in 8 portions over 1.3 hours, rinsed in with 2×5
L portions of ethyl acetate, allowing the mixture to warm to −1° C. by
the end of the addition. The mixture was gradually warmed to 1° C. for
30 minutes, then to 20° C. over 2 hours, and maintained at (20˜25)° C.
for 5 hours. The reaction mixture was washed twice with water: the first
time adding 66 L water, stirring at room temperature for 40 minutes,
allowing the phases to separate for 30 minutes, then removing the
aqueous phase; the second time adding 68 L water, bringing the pH to 1.9
with the addition of 0.45 L 36% hydrochloric acid, stirring at room
temperature for 35 minutes, allowing the phases to separate for 1 hour,
then removing the aqueous phase. The organic phase was then heated to
38° C., and the pressure reduced to about 0.25 bar until no further gas
was released, then to about (0.07-0.1) bar and solvents removed by
distillation until 266 L of distillate had been removed. Four cycles of
addition of 45 L ethyl acetate and removal of 45 L solvent by
distillation were performed, and the water content of the remaining
mixture was checked to ensure that it was below 0.1%. With the mixture
at 36° C., 194 L heptanes was added, maintaining the temperature about
36° C., and held at that temperature for 2.3 hours. A further 194 L
heptanes was added, allowing the temperature to cool to 30° C., and the
temperature then reduced to −1° C. over 2.3 hours and then to −5° C.
over 1 hour, and N-BOC-S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester
recovered by filtration, washing twice with 30 L each of heptanes at −5°
C., giving 85 Kg (63 Kg dry basis)
N-BOC-S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester. Without
stirring, the damp N-BOC-S-benzyl-L-cysteinyl-D-phenylglycine ethyl
ester was loaded into an 800 L jacketed glass-lined reactor, followed by
257 L ethyl acetate. Stirring was started and the temperature brought
to 22° C., then the nitrogen purge stopped and 12.2 Kg hydrogen chloride
gas was added through an immersion tube over 1.8 hours, allowing the
temperature to increase to 38° C. The temperature was increased to 41°
C., and the mixture held at that temperature for 9 hours. About 280 L of
solvents were removed by distillation at that temperature and a
pressure of (0.2˜0.1) bar over about 2 hours. Two cycles of addition of
ethyl acetate and removal of solvent by distillation were performed,
using 52 L in the first cycle and 77 L in the second cycle, and the
viscous solution of S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester
hydrochloride (3) in ethyl acetate, 148 Kg, was cooled to room
temperature and filtered into a storage drum.Example 2Preparation of
N-BOC-L-glutamic acid α-ethyl ester (6)Without stirring, 41 Kg
N-BOC-L-glutamic acid γ-benzyl ester (4) was added to an 800 L jacketed
glass-lined reactor, followed by 2.5 L water and 123 L ethyl acetate.
The mixture was then stirred until the N-BOC-L-glutamic acid γ-benzyl
ester completely dissolved, keeping the temperature below 15° C.
Potassium carbonate fine powder, 23.4 Kg, was added in five batches, and
the mixture then heated to 55° C. and maintained at that temperature
for 40 minutes, giving a heterogeneous and completely fluid mixture.
Diethyl sulfate, 26.2 Kg, was added over 2 hours, and rinsed in with 5 L
ethyl acetate, with the temperature remaining at about 52° C. The
nitrogen purge was stopped and a solution of 20 Kg ammonium chloride in
73 L water at room temperature added over 2 hours to the mixture,
maintaining the temperature near 50° C., then rinsing in with 10 L
water. Nitrogen purging was resumed, and the mixture was maintained at
about 50° C. for 3 hours, then lowered to about 45° C., the stirring
stopped, the phases allowed to separate for 30 minutes, and the lower,
aqueous, phase removed. The organic phase, containing N-BOC-L-glutamic
acid γ-benzyl α-ethyl ester (5), was washed three times with water, each
time adding 41 L water, stirring at room temperature for 30 minutes,
allowing the phases to separate for 30 minutes, then removing the
aqueous phase. The organic phase was heated to 35° C., and the pressure
reduced, starting at about 0.2 bar and reducing as necessary until 82 Kg
solvent had been removed by distillation, leaving about (70˜80) L of
slightly opalescent solution. This solution was heated to 53° C., and
102 L heptanes was added, maintaining the same temperature. The solution
was then filtered, rinsing with a further 13 L heptanes, then cooled to
32° C. to cause crystallization and maintained at that temperature for 1
hour. A further 66 L heptanes was added, and the mixture cooled to 22°
C. and held for 1 hour, then cooled to −5° C. and held for another 1
hour. The mixture was then filtered to isolate the N-BOC-L-glutamic acid
γ-benzyl α-ethyl ester (5), which was washed twice, each time with 25 L
heptanes cooled to (−5˜0)° C., and dried under vacuum at 40° C., giving
39.3 Kg N-BOC-L-glutamic acid γ-benzyl α-ethyl ester (5).A 4000 L
hydrogenator was purged with nitrogen, then under nitrogen sweep and no
stirring loaded with 39.2 Kg N-BOC-L-glutamic acid γ-benzyl α-ethyl
ester (5), 2.0 Kg 5% palladium on carbon, and 432 L ethyl acetate, and
purged (3 bar) and decompressed (0.2 bar) twice with nitrogen and twice
with hydrogen. Stirring was begun and the mixture heated to (37±2)° C.,
hydrogenated at that temperature under 2.8 bar hydrogen pressure until
no further hydrogen absorption occurred, then held under 2.8 bar
hydrogen pressure for 12 hours. Completion of hydrogenation was
confirmed by thin-layer chromatography of a sample. The mixture was
cooled to 28° C., the hydrogen purged from the hydrogenator, and the
hydrogenator purged (2 bar) and decompressed (0.2 bar) twice with
nitrogen. The mixture was filtered through a filter precoated with 10 Kg
powdered cellulose in 200 L ethyl acetate, then the filter washed with
the ethyl acetate used to form the precoat, giving a total of 626 Kg of a
dilute ethyl acetate solution containing 29.5 Kg N-BOC-L-glutamic acid
α-ethyl ester (6). This was distilled at (35˜40)° C. and (0.16˜0.18) bar
to give 67 L of concentrated solution, then 29 L of ethyl acetate added
and the solution redistilled to again give 67 L of concentrated
solution.Example 3Preparation of Ezatiostat HydrochlorideThe
concentrated solution of N-BOC-L-glutamic acid α-ethyl ester (6), 61.2
Kg (containing 27.8 Kg N-BOC-L-glutamic acid α-ethyl ester), was added
to a 600 L jacketed glass-lined reactor, rinsed in with 5 L ethyl
acetate, then cooled to 14° C. NMM, 10.8 Kg, was added over 50 minutes
and rinsed in with 5 L ethyl acetate, then stirring stopped, giving an
ethyl acetate solution of N-BOC-L-glutamic acid α-ethyl ester NMM salt.
Ethyl acetate, 475 L, was added to a 1300 L cooled jacketed glass-lined
reactor, followed by 14.5 Kg isobutyl chloroformate, rinsed in with 2×10
L ethyl acetate, and the mixture cooled to −11° C. The N-BOC-L-glutamic
acid α-ethyl ester NMM salt solution was added to the 1300 L reactor
over 1.3 hours, its reactor rinsed with 10 L ethyl acetate, and the
rinse solution added to the 1300 L reactor, then stirred for an
additional 30 minutes, while maintaining the temperature at about −13°
C.S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride (3) in
ethyl acetate, 112 Kg (containing 41.3 Kg
S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride) was
added in 4 portions over 45 minutes, and rinsed in with 5 L ethyl
acetate, followed by 10.8 Kg NMM in 8 portions over 1.3 hours, rinsed in
with 2×5 L portions of ethyl acetate, allowing the mixture to warm to
−4° C. by the end of the addition. The mixture was gradually warmed to
30° C. over 2 hours, and maintained at (30˜35)° C. for 2 hours. The
reaction mixture was washed twice with water: the first time adding 100 L
water, heating to 41° C., allowing the phases to separate for 30
minutes, then removing the aqueous phase; the second time adding 100 L
water, bringing the pH to 2.0 with the addition of 0.8 L 36%
hydrochloric acid, stirring at 43° C. for 30 minutes, allowing the
phases to separate for 1 hour, then removing the aqueous phase. The
organic phase was then heated to 42° C., and the pressure reduced to
about 0.25 bar until no further gas was released and solvents removed by
distillation until 495 L of distillate had been removed. Four cycles of
addition of 120 L ethyl acetate and removal of 120 L solvent by
distillation were performed, and the water content of the remaining
mixture was checked to ensure that it was below 0.1%. With the mixture
at 42° C., 610 L of ethyl acetate was added, maintaining the temperature
about 41° C., then heating to 58° C. to ensure dissolution. The
solution was filtered, rinsing the filter with 18 L ethyl acetate, and
the solution allowed to cool to 22° C. The nitrogen purge was stopped
and 22.2 Kg hydrogen chloride gas was added through an immersion tube
over 2 hours, then the mixture held at that temperature for 2 hours. The
mixture was heated to 31° C. over 1.5 hours, and held at about that
temperature for 15.5 hours. Solvents were removed by distillation at 33°
C. and a pressure of about 0.13 bar over about 1.5 hours to give a
volume of concentrated solution of about 630 L. Ethyl acetate, 100 L,
was added, and the mixture cooled to 25° C. and held at that temperature
for 30 minutes. The crude ezatiostat hydrochloride was recovered by
filtration and washed with 30 L ethyl acetate, giving 113 Kg damp crude
ezatiostat hydrochloride, which was dried at 40° C. under vacuum for 24
hours to give 52.8 Kg dry crude ezatiostat hydrochloride.Example
4Crystallization of Ezatiostat Hydrochloride to Form Pure Crystalline
Ezatiostat Hydrochloride Ansolvate Form D61.5 Kg crude ezatiostat
hydrochloride was added to a reactor at room temperature, followed by
399 liter (L) ethanol, and this mixture was heated to 68° C. to
completely dissolve the ezatiostat hydrochloride, filtered, then allowed
to cool to 65° C. and checked for clarity and the absence of
crystallization. About 1.3 Kg of ezatiostat hydrochloride ansolvate form
D was suspended in 9 L of ethyl acetate, and about one-half of this
suspension was added to the ethanol solution. The mixture was cooled to
63° C. and the second half of the suspension added to the mixture. The
resulting mixture was cooled gradually to 45° C., 928 L ethyl acetate
was added, and the mixture was cooled to 26° C. and held at about that
temperature for about 5 hours, then cooled to −2° C. The mixture,
containing crystalline ezatiostat hydrochloride ansolvate, was filtered,
and the residue washed twice with 65 L of chilled (0-5° C.) ethyl
acetate. The crystalline ezatiostat hydrochloride ansolvate was dried at
30° C. for 48 hours, then cooled to room temperature and sieved.
Analysis of the material by DSC and XRPD confirmed its identity as
crystalline ezatiostat hydrochloride ansolvate, and Karl Fischer
analysis showed a water content of 0.1%.Example 5Purifying Ezatiostat
Hydrochloride Crystals to Form Pure Crystalline Ezatiostat Hydrochloride
Ansolvate Form DCrude ezatiostat hydrochloride, 51.4 Kg, was added to a
600 L jacketed glass-lined reactor at room temperature, followed by 334
L of ethanol. The mixture was heated to 68° C. to completely dissolve
the ezatiostat hydrochloride. The resulting solution was filtered into a
1300 L jacketed glass-lined reactor, and an additional 27 L ethanol
warmed to 66° C. used to rinse the first reactor into the second reactor
through the filter. The resulting solution in the second reactor was
cooled to 63° C. and checked for complete dissolution; then 4 L of a
seeding suspension of crystalline ezatiostat hydrochloride ansolvate in
ethyl acetate was added, and the mixture cooled to 60° C. The remaining 4
L of the seeding suspension was added, and the mixture cooled to 47° C.
over 2 hours. The solids in the mixture were shown by DSC to contain
more than one form of ezatiostat hydrochloride, so the stages of heating
to dissolution, cooling, and adding seeding suspension (this time 2×2
L), were repeated, then the mixture cooled to 41° C. This time the
solids in the mixture were confirmed by DSC to be crystalline ezatiostat
hydrochloride ansolvate. Ethyl acetate, 776 L, was added, and the
mixture was cooled to 25° C. over 1.3 hours and further to 20° C. over
an additional 5 hours, then cooled to −3° C. The mixture, containing
crystalline ezatiostat hydrochloride ansolvate, was filtered and the
solids washed twice with 54 L each of chilled (−5˜0)° C. ethyl acetate.
The damp solids of crystalline ezatiostat hydrochloride ansolvate, 70
Kg, were dried in a vacuum oven at 25° C. for 16 hours, 35° C. for 7
hours, then at room temperature for 1 hour, then sieved. The crystalline
ezatiostat hydrochloride ansolvate, 44.2 Kg, had a loss on drying at
40° C. under vacuum for 2 hours of 0.09%, and a water content by Karl
Fischer analysis of 0.09%.
……………………………………………………………
U.S. Pat. No. 5,763,570
https://www.google.com/patents/US5763570
……………………………..
http://www.google.com/patents/WO2011156025A1?cl=en
Example 1. Preparation Of Ezatiostat Hydrochloride Ansolvate By Slurrying
[0082] Ezatiostat hydrochloride monohydrate was added to methyl tert-butyl ether at room temperature in excess, so that undissolved solids were present. The mixture was then agitated in a sealed vial at room temperature for 4 days, and the solids were then isolated by suction filtration. XRPD analysis of the solids established that the isolated solids were ezatiostat hydrochloride ansolvate.
[0083] Ezatiostat hydrochloride monohydrate was added to hexanes at 60 °C in excess, so that undissolved solids were present. The mixture was then agitated in a sealed vial at 60 °C for 4 days, and the solids were then isolated by suction filtration. XRPD analysis of the solids established that the isolated solids were ezatiostat hydrochloride ansolvate.
Example 2. Preparation Of Crystalline Ezatiostat Hydrochloride Ansolvate By Heating
[0084] DSC of crystalline ezatiostat hydrochloride monohydrate showed the pattern in FIG. 1, as discussed in paragraph above. Hot stage microscopy showed an initial melt followed by a recrystallization at 153 °C and a final melt at 166 °C. VT-XRPD, where XRPD patterns were obtained at 28 °C, 90 °C, and 160 °C during heating, and 28 °C after cooling of the formerly heated material, showed the presence of ezatiostat hydrochloride monohydrate at 28 °C and 90 °C during heating and of crystalline ezatiostat hydrochloride ansolvate at 160 °C and 28 °C after cooling of the formerly heated material. This confirmed that the transition at around 153/156 °C was a conversion of ezatiostat hydrochloride monohydrate form A to crystalline ezatiostat hydrochloride ansolvate form D and that the final DSC endothermic peak at about 177 °C (166 °C in the hot stage microscopy) was due to the melting of crystalline ezatiostat hydrochloride ansolvate. This was further confirmed by XRPD of the TG-IR material, where XRPD patterns obtained at room temperature both before and after heating to about 160 °C showed that the material before heating was form A and that the material after heating was form D ansolvate. DSC of crystalline ezatiostat hydrochloride ansolvate prepared by recrystallization showed the pattern in FIG. 5, with only the endothermic peak at about
177 °C followed by a broad endotherm at about (205 – 215) °C. Accordingly, the presence of the DSC endothermic peak at about 177 °C, for example at (177±2) °C, when measured under the conditions described above, is considered characteristic of crystalline ezatiostat hydrochloride ansolvate, and the substantial absence of thermal events at temperatures below this is considered indicative of the absence of other forms of ezatiostat hydrochloride
………………………………..
http://www.google.com/patents/WO2013082462A1?cl=en
Ezatiostat, also known as TLK199 or TER 199, is a compound of the formula:
[0003] Ezatiostat has been shown to induce the differentiation of HL-60 promyelocytic leukemia cells in vitro, to potentiate the activity of cytotoxic agents both in vitro and in vivo, and to stimulate colony formation of all three lineages of hematopoietic progenitor cells in normal human peripheral blood. In preclinical testing, ezatiostat has been shown to increase white blood cell production in normal animals, as well as in animals in which white blood cells were depleted by treatment with cisplatin or fluorouracil. Similar effects may provide a new approach to treating myelodysplasia syndrome (MDS).
[0004] Many conditions, including MDS, a form of pre-leukemia in which the bone marrow produces insufficient levels of one or more of the three major blood elements (white blood cells, red blood cells, and platelets), are characterized by depleted bone marrow.
Myelosuppression, which is characterized by a reduction in blood cell levels and in a reduction of new blood cell generation in the bone marrow, is also a common, toxic effect of many standard chemotherapeutic drugs.
[0005] Ezatiostat hydrochloride is the hydrochloride acid addition salt of ezatiostat.
Ezatiostat hydrochloride in a liposomal injectable formulation was studied in a clinical trial for the treatment of MDS, and results from this trial, reported by Raza et al., J. Hem. One, 2:20 (published online 13 May 2009), demonstrated that administration of TLK199 was well tolerated and resulted in multi-lineage hematologic improvement. Ezatiostat hydrochloride in a tablet formulation has been evaluated in a clinical trial for the treatment of MDS, as reported by Raza et al, Blood, 113:6533-6540 (prepublished online 27 April 2009) and a single-patient report by Quddus et al, J. Hem. One, 3:16 (published online 23 April 2010), and is currently being evaluated in clinical trials for the treatment of MDS and for severe chronic idiopathic neutropenia.
…………………………………..
http://www.google.com/patents/WO2013082462A1?cl=en
Example 1
[0048] 80 mg of crystalline ezatiostat hydrochloride was placed in a round bottom flask and dissolved in 25 mL of methanol. The solvent was then evaporated on a rotary evaporation apparatus under reduced pressure at 30 °C. After 30 minutes, the solid sample was removed from the round bottom flask and stored in a sealed vial at 2 °C in a refrigerator. Analysis of this sample was carried out within 24 hours of removing it from the rotary evaporation apparatus.
[0049] The resulting amorphous material was analyzed by 1H NMR, 13C NMR, DSC, and X-Ray powder diffraction experiments. The DSC conditions were 30 to 300 °C at 10 °C/ min using 7 mg of the amorphous material. The X-Ray powder diffraction was taken at 0-60 of 2theta. The crystalline ezatiostat hydrochloride was also analyzed.
Phase II trials: Ezatiostat
is the first GSTP1-1 inhibitor shown to cause clinically significant
and sustained reduction in RBC transfusions, transfusion independence,
and multilineage responses in MDS patients. The tolerability and
activity profile of ezatiostat may offer a new treatment option for patients with MDS. (source: Cancer. 2012 Apr 15;118(8):2138-47.)
References
|
1: Galili N, Tamayo P, Botvinnik OB, Mesirov JP,
Brooks MR, Brown G, Raza A. Prediction of response to therapy with
ezatiostat in lower risk myelodysplastic syndrome. J Hematol Oncol. 2012
May 6;5:20. doi: 10.1186/1756-8722-5-20. PubMed PMID: 22559819; PubMed
Central PMCID: PMC3407785.
2: Raza A, Galili N, Mulford D, Smith SE, Brown GL, Steensma DP,
Lyons RM, Boccia R, Sekeres MA, Garcia-Manero G, Mesa RA. Phase 1
dose-ranging study of ezatiostat hydrochloride in combination with
lenalidomide in patients with non-deletion (5q) low to intermediate-1
risk myelodysplastic syndrome (MDS). J Hematol Oncol. 2012 Apr 30;5:18.
doi: 10.1186/1756-8722-5-18. PubMed PMID: 22546242; PubMed Central
PMCID: PMC3416694.3: Lyons RM, Wilks ST, Young S, Brown GL. Oral ezatiostat HCl (Telintra®, TLK199) and idiopathic chronic neutropenia (ICN): a case report of complete response of a patient with G-CSF resistant ICN following treatment with ezatiostat, a glutathione S-transferase P1-1 (GSTP1-1) inhibitor. J Hematol Oncol. 2011 Nov 2;4:43. doi: 10.1186/1756-8722-4-43. PubMed PMID: 22047626; PubMed Central PMCID: PMC3235963.
4: Raza A, Galili N, Smith SE, Godwin J, Boccia RV, Myint H, Mahadevan D, Mulford D, Rarick M, Brown GL, Schaar D, Faderl S, Komrokji RS, List AF, Sekeres M. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer. 2012 Apr 15;118(8):2138-47. doi: 10.1002/cncr.26469. Epub 2011 Sep 1. PubMed PMID: 21887679.
5: Quddus F, Clima J, Seedham H, Sajjad G, Galili N, Raza A. Oral Ezatiostat HCl (TLK199) and Myelodysplastic syndrome: a case report of sustained hematologic response following an abbreviated exposure. J Hematol Oncol. 2010 Apr 23;3:16. doi: 10.1186/1756-8722-3-16. PubMed PMID: 20416051; PubMed Central PMCID: PMC2873355.
6: Steensma DP. Novel therapies for myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010 Apr;24(2):423-41. doi: 10.1016/j.hoc.2010.02.010. Review. PubMed PMID: 20359635.
7: D’Alò F, Greco M, Criscuolo M, Voso MT. New treatments for myelodysplastic syndromes. Mediterr J Hematol Infect Dis. 2010 Aug 11;2(2):e2010021. doi: 10.4084/MJHID.2010.021. PubMed PMID: 21415972; PubMed Central PMCID: PMC3033133.
8: Raza A, Galili N, Callander N, Ochoa L, Piro L, Emanuel P, Williams S, Burris H 3rd, Faderl S, Estrov Z, Curtin P, Larson RA, Keck JG, Jones M, Meng L, Brown GL. Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J Hematol Oncol. 2009 May 13;2:20. doi: 10.1186/1756-8722-2-20. PubMed PMID: 19439093; PubMed Central PMCID: PMC2694211.
9: Raza A, Galili N, Smith S, Godwin J, Lancet J, Melchert M, Jones M, Keck JG, Meng L, Brown GL, List A. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood. 2009 Jun 25;113(26):6533-40. doi: 10.1182/blood-2009-01-176032. Epub 2009 Apr 27. PubMed PMID: 19398716.
| WO2013082462A1 * | Nov 30, 2012 | Jun 6, 2013 | Telik, Inc. | Amorphous ezatiostat ansolvate |
| US20120251496 * | Mar 20, 2012 | Oct 4, 2012 | Telik, Inc. | Ezatiostat for treating multiple myeloma |
Figure 1.
Known Yes1 kinase inhibitors, dasatinib and saracatinib.
Table 1. Select Yes1 kinase inhibitors from a HTS and their corresponding clinical phase, known targets, and IC50 values
| Compound name and NCGC ID | Structure | Clinical phase | Known targets | Yes1 IC50 (nM) |
|---|---|---|---|---|
| Dasatinib (1) NCGC00181129 |
Approved | Lyn, PDGFR, KIT, Lck, BTK, Bcr–Abl, Fyn, Yes1, c-Src |
0.5 (<1.0)a | |
| Saracatinib (2) NCGC00241099 |
Phase II/III | c-Src, Bcr–Abl, Yes1, Lck | 6.2 (0.70)a | |
| AEE-788 (3) NCGC00263149 |
Phase I/II | EGFR, HER-2, VEGFR-2 | 17.5 (13.1)a | |
| Dovitinib (4) NCGC00249685 |
Phase III | FGFR, EGFR, PDGFR, VEGFR-1,2 | 31 (1.4)a | |
| DCC-2036 (5) NCGC00263172 |
Phase I/II | Bcr–Abl, Tie-2, Lyn, FLT3, VEGFR-2 | 2.5 (1.5)a | |
| SGI-1776 (6) NCGC00263186 |
Discontinued | Pim-1, FLT3 | 2670 (240)a | |
| AMG-Tie-2-1 (7) NCGC00263199 |
Preclinical | Tie-2 | 8.7 (22.0)a | |
| AZ-23 (8) NCGC00250381 |
Preclinical | Trk | 39.1 (3.0)a | |
| Dorsomorphin (9) NCGC00165869 |
Preclinical | AMPK, BMPR, TGFβ Receptor | 195.9 (29.8)a | |
| AZ-628 (10) NCGC00250380 |
Preclinical | Raf Kinase B,C | 348.3 (51.2)a |
- http://www.sciencedirect.com/science/article/pii/S0960894X13006677
- Data in parentheses were gathered by Reaction Biology Corp. using a [γ-33P]-ATP radiolabeled enzyme activity assay at an ATP concentration of 10 μM (www.reactionbiology.com).
6
DACINOSTAT
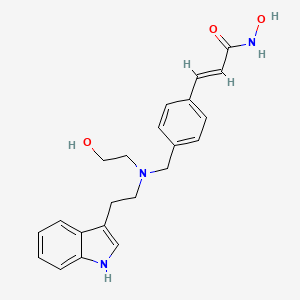
Dacinostat (LAQ-824, NVP-LAQ824,)
((E)-N-hydroxy-3-[4-[[2-hydroxyethyl-[2-(1 H-indol-3-yl)ethyl]amino]methyl]phenyl]prop-2-enamide
(2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide
404951-53-7
Dacinostat, also known as LAQ824, is a hydroxamate histone deacetylase inhibitor with potential anticancer activity. LAQ824 sensitized nonsmall cell lung cancer to the cytotoxic effects of ionizing radiation. LAQ824 reduced clonogenic survival of the H23 and H460 cell lines five-fold compared with controls and four-fold compared with either agent alone (P<0.001). In phase I trials, LAQ824 was well tolerated at doses that induced accumulation of histone acetylation, with higher doses inducing changes consistent with HSP90 inhibition.
NVP-LAQ824 inhibits histone deacetylase enzymatic activities in vitro and transcriptionally activated the p21 promoter in reporter gene assays. When tested on a variety of solid tumour cell lines, NVP-LAQ824 exhibited selective anti-proliferative effects, inducing cell growth inhibition in some, while inducing cell death in others. To induce cell death, a minimum of 16 h exposure to NVP-LAQ824 is required. Flow cytometry studies revealed that both tumour cell lines and normal diploid fibroblasts arrested in the G2/M phase of the cell cycle after compound treatment. However, an increased sub-G1 population at 48 h (reminiscent of apoptotic cells) was only observed in the cancer cell lines.
Annexin V staining data confirmed that NVP-LAQ824 induced apoptosis in tumour cells, but not in normal cells. To relate HDAC inhibition to the anti-proliferative effects of NVP-LAQ824, expression of HDAC 1 was inhibited using antisense and this was sufficient to activate p21 expression, hypophosphorylate Rb and inhibit cell growth. Furthermore, tumour cells treated with NVP-LAQ824 caused acetylation of HSP90 and degradation of its cargo oncoproteins. Finally, NVP-LAQ824 exhibited antitumour effects in a xenograft animal model.
To determine if NVP-LAQ824 inhibited histone deacetylases in vivo, tumours treated with the drug were immunoblotted with an antibody specific for acetylated histones H3 and H4 and the results indicated increased histone H3 and 114 acetylation levels in NVP-LAQ824 treated cancer cells. Together, our data indicated that the activity of NVP-LAQ824 was consistent with its intended mechanism of action. This novel HDAC inhibitor is currently in clinical trials as an anticancer agent. see: http://www.ncbi.nlm.nih.gov/pubmed/15171259.
Reversible acetylation of histones is a major regulator of gene expression that acts by altering accessibility of transcription factors to DNA. In normal cells, histone deacetylase (HDA) and histone acetyltrasferase together control the level of acetylation of histones to maintain a balance. Inhibition of HDA results in the accumulation of hyperacetylated histones, which results in a variety of cellular responses.
Inhibitors of HDA have been studied for their therapeutic effects on cancer cells. For example, butyric acid and its derivatives, including sodium phenylbutyrate, have been reported to induce apoptosis in vitro in human colon carcinoma, leukemia and retinoblastoma cell lines. However, butyric acid and its derivatives are not useful pharmacological agents because they tend to be metabolized rapidly and have a very short half-life in vivo. Other inhibitors of HDA that have been widely studied for their anti-cancer activities are trichostatin A and trapoxin. Trichostatin A is an antifungal and antibiotic and is a reversible inhibitor of mammalian HDA. Trapoxin is a cyclic tetrapeptide, which is an irreversible inhibitor of mammalian HDA.
Although trichostatin and trapoxin have been studied for their anti-cancer activities, the in vivo instability of the compounds makes them less suitable as anti-cancer drugs. There remains a need for an active compound that is suitable for treating tumors, including cancerous tumors, that is highly efficacious and stable
……………………….
PATENT
WO 200222577
Proc Am Assoc Cancer Res 2002,43Abst 3671
The esterification of 4-formylcinnamic acid (I) with methanol and HCl gives the methyl ester (II), which can be obtained by Heck coupling of 4-bromobenzaldehyde (III) with methyl acrylate (IV). The reductocondensation of (II) with tryptamine (V) by means of NaBH(OAc)3 in dichloroethane yields the secondary amine (VI), which is alkylated with 2-(tert-butyldimethylsilyloxy)ethyl bromide (VII) by means of DIEA in DMSO to afford the tertiary amine (VIII). The reaction of the methyl ester group of (VIII) with KOH and hydroxylamine in methanol provides the silylated hydroxamic acid (IX), which is finally deprotected with TFA in water.
References
|
1: Wang H, Cheng F, Woan K, Sahakian E, Merino O, Rock-Klotz J, Vicente-Suarez I, Pinilla-Ibarz J, Wright KL, Seto E, Bhalla K, Villagra A, Sotomayor EM. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL-10. J Immunol. 2011 Apr 1;186(7):3986-96. doi: 10.4049/jimmunol.1001101. Epub 2011 Mar 2. PubMed PMID: 21368229.
2: Schwarz K, Romanski A, Puccetti E, Wietbrauk S, Vogel A, Keller M, Scott JW, Serve H, Bug G. The deacetylase inhibitor LAQ824 induces notch signalling in haematopoietic progenitor cells. Leuk Res. 2011 Jan;35(1):119-25. doi: 10.1016/j.leukres.2010.06.024. Epub 2010 Jul 31. PubMed PMID: 20674020.
3: Cho YS, Whitehead L, Li J, Chen CH, Jiang L, Vögtle M, Francotte E, Richert P, Wagner T, Traebert M, Lu Q, Cao X, Dumotier B, Fejzo J, Rajan S, Wang P, Yan-Neale Y, Shao W, Atadja P, Shultz M. Conformational refinement of hydroxamate-based histone deacetylase inhibitors and exploration of 3-piperidin-3-ylindole analogues of dacinostat (LAQ824). J Med Chem. 2010 Apr 8;53(7):2952-63. doi: 10.1021/jm100007m. PubMed PMID: 20205394.
4: Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, Craft N, Economou JS, Marincola FM, Wang E, Ribas A. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009 Nov 15;69(22):8693-9. doi: 10.1158/0008-5472.CAN-09-1456. Epub 2009 Oct 27. PubMed PMID: 19861533; PubMed Central PMCID: PMC2779578.
5: Ellis L, Bots M, Lindemann RK, Bolden JE, Newbold A, Cluse LA, Scott CL, Strasser A, Atadja P, Lowe SW, Johnstone RW. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009 Jul 9;114(2):380-93. doi: 10.1182/blood-2008-10-182758. Epub 2009 Apr 21. PubMed PMID: 19383971.
6: de Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, Mita M, Shaw H, Workman P, Kaye S, Rowinsky EK, Aherne W, Atadja P, Scott JW, Patnaik A. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res. 2008 Oct 15;14(20):6663-73. doi: 10.1158/1078-0432.CCR-08-0376. PubMed PMID: 18927309.
7: Chung YL, Troy H, Kristeleit R, Aherne W, Jackson LE, Atadja P, Griffiths JR, Judson IR, Workman P, Leach MO, Beloueche-Babari M. Noninvasive magnetic resonance spectroscopic pharmacodynamic markers of a novel histone deacetylase inhibitor, LAQ824, in human colon carcinoma cells and xenografts. Neoplasia. 2008 Apr;10(4):303-13. PubMed PMID: 18392140; PubMed Central PMCID: PMC2288545.
8: Cuneo KC, Fu A, Osusky K, Huamani J, Hallahan DE, Geng L. Histone deacetylase inhibitor NVP-LAQ824 sensitizes human nonsmall cell lung cancer to the cytotoxic effects of ionizing radiation. Anticancer Drugs. 2007 Aug;18(7):793-800. PubMed PMID: 17581301.
9: Kato Y, Salumbides BC, Wang XF, Qian DZ, Williams S, Wei Y, Sanni TB, Atadja P, Pili R. Antitumor effect of the histone deacetylase inhibitor LAQ824 in combination with 13-cis-retinoic acid in human malignant melanoma. Mol Cancer Ther. 2007 Jan;6(1):70-81. PubMed PMID: 17237267.
10: Leyton J, Alao JP, Da Costa M, Stavropoulou AV, Latigo JR, Perumal M, Pillai R, He Q, Atadja P, Lam EW, Workman P, Vigushin DM, Aboagye EO. In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Cancer Res. 2006 Aug 1;66(15):7621-9. PubMed PMID: 16885362.
7
ITF2357, UNII-5P60F84FBH, ITF-2357, Gavinostat,
[6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate,
diethyl-[6-(4-hydroxycarbamoyl-phenylcarbamoyloxymethyl)-naphthalen-2-yl-methyl]-amine
4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]benzohydroxamic acid
CAS 497833-27-9 FREE BASE
199657-29-9 HCL SALT
Molecular Formula: C24H27N3O4
Molecular Weight: 421.48888 g/mol
PHASE 2 Italfarmaco (INNOVATOR)
DESCRIBED IN U.S. Pat. No. 6,034,096 or in U.S. Pat. No. 7,329,689.
Givinostat (INN[1]) or gavinostat (originally ITF2357) is a histone deacetylase inhibitor with potential anti-inflammatory, anti-angiogenic, and antineoplastic activities.[2] It is a hydroxamate used in the form of its hydrochloride.
Givinostat is in numerous phase II clinical trials (including for relapsed leukemias and myelomas),[3] and has been granted orphan drug designation in the European Union for the treatment of systemic juvenile idiopathic arthritis[4] and polycythaemia vera.[5]
In 2010, orphan drug designation was assigned in the E.U. for the treatment of systemic-onset juvenile idiopathic arthritis and for the treatment of polycythemia vera. In 2013, this designation was assigned by the FDA for the treatment of Duchenne’s muscular dystrophy and for the treatment of Becker’s muscular dystrophy.
ITF2357 was discovered at Italfarmaco of Milan, Italy. It was patented in 1997 and first described in the scientific literature in 2005.[6][7]
Givinostat hydrochloride, an orally active, synthetic inhibitor of histone deacetylase, is being evaluated in several early clinical studies at Italfarmaco, including studies for the treatment of myeloproliferative diseases, polycythemia vera, Duchenne’s muscular dystrophy and periodic fever syndrome. The company was also conducting clinical trials for the treatment of Crohn’s disease and chronic lymphocytic leukemia; however, the trials were terminated.
No recent development has been reported for research into the treatment of juvenile rheumatoid arthritis, for the treatment of multiple myeloma and for the treatment of Hodgkin’s lymphoma.
| Patent | Submitted | Granted |
|---|---|---|
| Monohydrate hydrochloride of the 4-hydroxycarbamoyl-phenyl)-carbamic acid (6-diethylaminomethyl-naphtalen-2-yl) ester [US7329689] | 2005-11-03 | 2008-02-12 |
Adverse effects
In clinical trials of givinostat as a salvage therapy for advanced Hodgkin’s lymphoma, the most common adverse reactions were fatigue (seen in 50% of participants), mild diarrhea or abdominal pain (40% of participants), moderate thrombocytopenia (decreased platelet counts, seen in one third of patients), and mild leukopenia (a decrease in white blood cell levels, seen in 30% of patients). One-fifth of patients experienced prolongation of the QT interval, a measure of electrical conduction in the heart, severe enough to warrant temporary suspension of treatment.[8]Mechanism of action
Givinostat inhibits class I and class II histone deacetylases (HDACs) and several pro-inflammatory cytokines. This reduces expression of tumour necrosis factor (TNF), interleukin 1α and β, and interleukin 6.[7]It also has activity against cells expressing JAK2(V617F), a mutated form of the janus kinase 2 (JAK2) enzyme that is implicated in the pathophysiology of many myeloproliferative diseases, including polycythaemia vera.[9][10] In patients with polycythaemia, the reduction of mutant JAK2 concentrations by givinostat is believed to slow down the abnormal growth of erythrocytes and ameliorate the symptoms of the disease.[5]
………………….
PATENT
http://www.google.co.in/patents/US7329689
Hydrochloride of (6-diethylaminomethyl-naphthalen-2-yl)-methyl ester of (4-hydroxycarbamoylphenyl)-carbamic acid (II)
has been described in U.S. Pat. No. 6,034,096 as a derivative of hydroxamic acid having anti-inflammatory and immunosuppressive activity, probably owing to the ability thereof to inhibit the production of pro-inflammatory cytokines. This compound is obtained according to Example 12 of the above-mentioned patent as an anhydrous, amorphous, hygroscopic, deliquescent solid which is difficult to handle.
The 4-(6-diethylaminomethyl-naphthalen-2-ylmethoxycarbonylamino)-benzoic acid can be prepared as described in Example 12, point C, of U.S. Pat. No. 6,034,096.
The acid (1.22 kg, 3 moles) was suspended in THF (19 l) and the mixture was agitated under nitrogen over night at ambient temperature. The mixture was then cooled to 0° C. and thionyl chloride (0.657 l, 9 moles) was added slowly, still under nitrogen, with the temperature being maintained below 10° C. The reaction mixture was heated under reflux for 60 minutes, DMF (26 ml) was added and the mixture was further heated under reflux for 60 minutes.
The solvent was evaporated under vacuum, toluene was added to the residue and was then evaporated. This operation was repeated twice, then the residue was suspended in THF (11.5 l) and the mixture was cooled to 0° C.
The mixture was then poured into a cold solution of hydroxylamine (50% aq., 1.6 l, 264 moles) in 5.7 l of water. The mixture was then cooled to ambient temperature and agitated for 30 minutes. 6M HCl was added until pH 2 was reached and the mixture was partially evaporated under vacuum in order to eliminate most of the THF. The solid was filtered, washed repeatedly with water and dissolved in a solution of sodium bicarbonate (2.5%, 12.2 l). The solution was extracted with 18.6 l of a mixture of THF and ethyl acetate (2:1 v/v). 37% HCl (130 ml) were added to the organic layer in order to precipitate the monohydrate of the (6-diethylaminomethyl-naphthalen-2-yl)-methyl ester hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid. If necessary, this operation can be repeated several times to remove any residues of the original acid.
Finally, the solid was dried under vacuum (approximately 30 mbar, 50° C.), producing 0.85 kg (60%) of compound (I).
HPLC purity: 99.5%; water content (Karl Fischer method): 3.8%; (argentometric) assay: 99.8%.
| Elemental analysis | |||||
| C % | H % | Cl % | N % | ||
| Calculated for | 60.56 | 6.35 | 7.45 | 8.83 | |
| C24H30ClN3O5 | |||||
| Found | 61.06 | 6.48 | 7.48 | 8.90 | |
…..
PATENThttp://www.google.co.in/patents/US20120302633
…………..
…………………..http://www.google.com/patents/US6034096
EXAMPLE 12
4-[6-(Diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]-benzohydroxamic acid hydrochloride
A. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (22.2 g, 115 mmol) was added to a solution of 2,6-naphthalenedicarboxylic acid (25 g, 115 mmol) and hydroxybenzotriazole (15.6 g, 115 mmol) in dimethylformamide (1800 ml) and the mixture was stirred at room temperature for 2 hours. Diethyl amine (34.3 ml, 345 mmol) was added and the solution was stirred overnight at room temperature. The solvent was then evaporated under reduced pressure and the crude was treated with 1N HCl (500 ml) and ethyl acetate (500 ml), insoluble compounds were filtered off and the phases were separated. The organic phase was extracted with 5% sodium carbonate (3×200 ml) and the combined aqueous solutions were acidified with concentrated HCl and extracted with ethyl acetate (3×200 ml). The organic solution was then washed with 1N HCl (6×100 ml), dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure yielding 18.5 g (Yield 60%) of pure 6-(diethylaminocarbonyl)-2-naphthalenecarboxylic acid; m.p.=122-124° C.1 H-NMR d 8.67 (s, 1H), 8.25-8.00 (m, 4H), 7.56 (d, 1H), 3.60-3.20 (m, 4H), 1.30-1.00 (m, 6H).
B. A solution of 6-(diethylaminocarbonyl)-2- naphthalenecarboxylic acid (18 g, 66 mmol) in THF (200 ml) was slowly added to a refluxing suspension of lithium aluminium hydride (7.5 g, 199 mmol) in THF (500 ml). The mixture was refluxed for an hour, then cooled at room temperature and treated with a mixture of THF (25 ml) and water (3.5 ml), with 20% sodium hydroxide (8.5 ml) and finally with water (33 ml). The white solid was filtered off and the solvent was removed under reduced pressure. Crude was dissolved in diethyl ether (200 ml) and extracted with 1N HCl (3×100 ml). The aqueous solution was treated with 32% sodium hydroxide and extracted with diethyl ether (3×100 ml). The organic solution was dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure yielding 12.7 g (79% yield) of pure 6-(diethylaminomethyl)-2-naphthalenemethanol as thick oil.
1 H-NMR d 7.90-7.74 (m, 4H), 7.49 (m, 2H), 5.32 (t, 1H, exchange with D2 O), 4.68 (d, 2H), 3.69 (s, 2H), 2.52 (q, 4H), 1.01 (t, 6H).
C. A solution of 6-(diethylaminomethyl)-2-naphthalene-methanol (12.5 g, 51 mmol) and N,N’-disuccinimidyl carbonate (13.2 g, 51 mmol) in acetonitrile (250 ml) was stirred at room temperature for 3 hours, then the solvent was removed and the crude was dissolved in THF (110 ml). This solution was added to a solution of 4-amino benzoic acid (7.1 g, 51 mmol) and sodium carbonate (5.5 g, 51 mmol) in water (200 ml) and THF (100 ml). The mixture was stirred overnight at room temperature, then THF was removed under reduced pressure and the solution was treated with 1N HCl (102 ml, 102 mmol). The precipitate was filtered, dried under reduced pressure, tritured in diethyl ether and filtered yielding 13.2 g (yield 64%) of pure 4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]-benzoic acid; m.p.=201-205° C. (dec.)
1 H-NMR d 10.26 (s, 1H), 8.13 (s, 1H), 8.05-7.75 (m, 6H), 7.63 (m, 3H), 5.40 (s, 2H), 4.32 (s, 2H), 2.98 (q, 4H), 1.24 (t, 6H).
D. A solution of 4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]benzoic acid (13.1 g, 32 mmol) and thionyl chloride (7 ml, 96 mmol) in chloroform (300 ml) was refluxed for 4 hours, then the solvent and thionyl chloride were evaporated. Crude was dissolved in chloroform (100 ml) and evaporated to dryness three times. Crude was added as solid to a solution of hydroxylamine hydrochloride (2.7 g, 39 mmol) and sodium bicarbonate (5.4 g, 64 mmol) and 1N sodium hydroxide (39 ml, 39 mmol) in water (150 ml) and THF (50 ml). The mixture was stirred overnight at room temperature, then THF was removed under reduced pressure and the aqueous phase was extracted with ethyl acetate (3×100 ml). The combined organic phases were dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure. Crude was dissolved in THF and treated with a 1.5 N etheric solution of HCl. The solid product was filtered and dried yielding 6 g (yield 41%) of pure 4-[6-(diethylaminomethyl)naphth-2-ylmethyloxycarbamoyl]benzohydroxamic acid hydrochloride as white solid; m.p.=162-165° C., (dec.)
1 H-NMR d 11.24 (s, 1H, exchange with D2 O), 10.88 (s, 1H, exchange with D2 O), 10.16 (s, 1H), 8.98 (bs, 1H, exchange with D2 O), 8.21 (s, 1H), 8.10-7.97 (m, 3H), 7.89 (d, 1H), 7.80-7.55 (m, 5H), 5.39 (s, 2H), 4.48 (d, 2H), 3.09 (m, 4H), 1.30 (t, 6H).
References
1
- World Health Organization (2010). “International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended INN: List 63″. WHO Drug Information 24 (1): 58–9.
- 2
- National Cancer Institute (2010). “Gavinostat”. NCI Cancer Dictionary. U.S. National Institutes of Health. Retrieved 2010-09-15.
- 3
- “Search results for ITF2357″. ClinicalTrials.gov.
- 4
- Committee for Orphan Medicinal Products (23 February 2010). “Public summary of opinion on orphan designation: Givinostat for the treatment of systemic-onset juvenile idiopathic arthritis”. European Medicines Agency. Retrieved 2010-09-15.
- 5
- Committee for Orphan Medicinal Products (3 March 2010). “Public summary of opinion on orphan designation: Givinostat for the treatment of polycythaemia vera”. European Medicines Agency.
- 6
- WO patent application 1997/043251, “Compounds with anti-inflammatory and immunosuppressive activities”, published 1997-11-20, assigned to Italfarmaco S.p.A.
- 7
- Leoni F, Fossati G. (2005). “The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo”. Molecular Medicine 11: 1. doi:10.2119/2006-00005.Dinarello. PMID 16557334.
- 8
- Tan J, Cang S, Ma Y, Petrillo RL, Liu D (2010). “Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents”. Journal of Hematology & Oncology 3: 5. doi:10.1186/1756-8722-3-5. PMID 20132536. Review.
- 9
- Vannucchi AM, Guglielmelli P, Pieri L, Antonioli E, Bosi A (2009). “Treatment options for essential thrombocythemia and polycythemia vera.” Expert Review of Hematology 2 (1): 41–55. doi:10.1586/17474086.2.1.41. Review.
- Guerini V, Barbui V, Spinelli O, et al. (April 2008). “The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F)”. Leukemia 22 (4): 740–7. doi:10.1038/sj.leu.2405049. PMID 18079739.
Further reading
- Job-Deslandre, C (January 2007). “Idiopathic juvenile-onset systemic arthritis”. Orphanet. Orphan number: ORPHA85414.
| US6034096 | 12 May 1997 | 7 Mar 2000 | Italfarmaco S.P.A. | Compounds with anti-inflammatory and immunosuppressive activities |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8518988 * | 3 Dec 2010 | 27 Aug 2013 | Chemi Spa | Polymorph of the hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid (6-dimethylamino methyl-2-naphthalenyl) ester |
| US20120302633 * | 3 Dec 2010 | 29 Nov 2012 | Chemi Spa | Novel polymorph of the hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid (6-dimethylamino methyl-2-naphthalenyl) ester |
| WO2011092556A1 | 3 Dec 2010 | 4 Aug 2011 | Chemi Spa | Novel polymorph of the hydrochloride of the (4-hydroxycarbamoyl-phenyl)-carbamic acid (6-dimethylamino methyl-2-naphtalenyl) ester |
8 TOPIROXOSTAT  Topiroxostat 托匹司他 FUJI YAKUHIN ........INNOVATOR Approved in japan PMDA JUNE 28 2013 Xanthine oxidase inhibitor FOR GOUT AND HYPERURICEMIA Launched - 2013, Fuji YakuhinSanwa, Topiloric Uriadec IUPAC Name: 4-(5-pyridin-4-yl-1H-1,2,4-triazol-3-yl)pyridine-2-carbonitrile CAS Registry Number: 577778-58-6 4 - [5 - (pyridin-4 - yl)-1H-1, 2,4 - triazol-3 - yl] pyridine-2 - carbonitrile (1) 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole 3-(3-cyano-4-pyridyl)-5-(4-pyridyl)-1,2,4-triazole Synonyms: 4-(5-PYRIDIN-4-YL-1H-1,2,4-TRIAZOL-3-YL)PYRIDINE-2-CARBONITRILE, AC1NRB9T, Topiroxostat (JAN/INN), DB01685, D09786, FYX-051 SK-0910 4-[5-PYRIDIN-4-YL-1H-[1,2,4]TRIAZOL-3-YL]-PYRIDINE-2-CARBONITRILE, C13H8N6 MF,248.2482 MW  TOPIROXOSTAT TOPIROXOSTAT托匹司他 A xanthine oxidase inhibitor used to treat gout and hyperuricemia.   FYX-051, TOPIROXOSTAT is a xanthine oxidase inhibitor. This agent was approved in Japan by Fuji Yakuhin and Sanwa for the treatment of gout and hyperuricemia in 2013 and launched at the same year. In 2009, the compound was licensed to Sanwa by Fuji Yakuhin in Japan for the codevelopment and commercialization of gout. The number of patients with hyperuricemia in Japan is reported to be 1.25 million and the number suffering from asymptomatic hyperuricemia is estimated to reach several millions. Hyperuricemia is becoming a popular disease. Presently, hyperuricemia and gout due to hyperuricemia are treated by improving the living environment and administering various drug therapies for each period when an attack of gout is predicted to occur (presymptomatic period), when an attack of gout occurs, or when an attack of gout subsides. That is, preventive therapy is conducted in the presymptomatic period by administering colchicines as well as controlling the daily living environment. When an attack occurs, drug therapy using non-steroidal or steroidal anti-inflammatory agents is mainly conducted. After the attack subsides, patients are given guidance to improve their lifestyle. When improvement is judged insufficient, an assessment is made as to whether hyperuricemia is caused by reduced excretion of uric acid or by increased production of uric acid followed by treatment with drugs, which exhibit a uricosuric effect, such as probenecid and benzbromarone, those which inhibit resorption of uric acid, such as sulfinpyrazone, those which improve acidurea conditions, such as citrates, and xanthine oxidase inhibitors which inhibit production of uric acid, such as allopurinol. Colchicine is said to be able to prevent about 90% of attacks through inhibiting chemotaxis and phagocytosis of leukocytes, such as neutrophils, if administration thereof has been completed within a few hours before the attack. Since colchicine has various adverse effects, however, the use thereof is limited to the minimum and it is therefore difficult to timely administer it. Accordingly, drug therapies are mainly adopted, but only allopurinol is available for the treatment of a disease caused by increased production of uric acid. However, a metabolite of allopurinol, oxypurinol, tends to accumulate and may cause calculi formation. Furthermore, this drug has been reported to induce adverse events such as rash, a decreased renal function and hepatitis, and it is not easy to administer. Examples of compounds having xanthine oxidase inhibiting activity that can be used for treating gout caused by increased production of uric acid and that are effective for hyperuricemia and gout due to hyperuricemia have been described in J. Medicinal Chemistry, 1975, Vol. 18, No. 9, pp. 895–900, Japanese Patent Publication No. 49-46622 and Japanese Patent Publication No. 50-24315, which disclose some 1,3,5-substituted or 3,5-substituted 1,2,4-triazole compounds. 4 - [5 - (pyridin-4 - yl)-1H-1, 2,4 - triazol-3 - yl] pyridine-2 - carbonitrile (1) has a xanthine oxidase inhibitory activity and serum uric acid level known as the agent that reduces (Patent Document 1). The method for producing the compound (1), for example, 2 by Reissert Henze reaction isonicotinic acid methyl N-oxide - is a cyano isonicotinate, and the hydrazide which is then, 4 - this condensed cyanopyridine After obtaining a hydrazide of isonicotinic acid N-oxide (Patent Document 1, Example 12) and method, a cyano group after introduction, 4 by Reissert Henze reaction - method of condensing a cyano pyridine is known (Patent Document 1, Example 39).Further, 4 - as a starting material cyano-N-oxide, a triazole ring after construction (Patent Document 3), Reissert Henze unprotected or (Patent Document 2) to protect the ring condensed with isonicotinic acid hydrazide method of obtaining the compound (1) by introducing a cyano group by the reaction have also been reported. The crystalline polymorph, yet the same molecule with the same chemical composition, the molecular arrangement in the crystal are different, and are different crystalline states. The pharmaceutical compounds having crystal polymorphism such the differences in physicochemical properties, affect pharmacological activity, solubility, bioavailability, stability and the like are known.Therefore, when the crystal polymorphism is present in a pharmaceutically useful compound, producing compounds of the crystalline form highly useful from polymorphs thereof is desirable.
WO 2003/064410 discloses WO 2005/009991 discloses Japanese Patent Publication No. 2005-41802
However,
4 of the above Patent Document - no description about the presence of
crystalline polymorph on carbonitrile - pyridine-2-[yl 5 - (pyridin-4 -
yl)-1H-1, 2,4 - - -3 triazol] It has not been, to these manufacturing
methods, it is disclosed a method for the purpose of improving the
chemical purity and yield, there is no description of the
crystallographic plane.Method of producing topiroxostat, useful for preventing or treating gout; and its intermediates. Picks up from WO2012060308, claiming the use of this topiroxostat for treating renal dysfunction. Along with the concurrently published WO2014017515, claiming crystalline Forms I and II of this compound, which, Fuji Yakuhin, in collaboration with Sanwa Kagaku, has developed and launched for the treatment of gout and hyperuricemia.WO-2014017516 Crystalline Forms I and II of topiroxostat, useful for preventing or treating gout. Along with the concurrently published WO2014017516, claiming a method of producing this compound. Picks up from WO2012060308, claiming a method of treating renal dysfunction using topiroxostat, which Fuji Yakuhin, in collaboration with Sanwa Kagaku, has developed and launched for the treatment of gout and hyperuricemia.WO-2014017515 novel 1,2,4-triazole compounds having an optionally substituted 2-cyanopyridin-4-yl group at 3-position and an optionally substituted aromatic group at 5-position inhibit a xanthine oxidase and are useful for treatment of gout and hyperuricemia, and have previously filed a patent application (Patent Document 1). The compounds can be prepared according to a method shown by the following reaction scheme:
SYNTHESIS     PATENT EP1650204A1
Preparation of 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole
SYNTHESIS US7074816 Example 12 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole 1) Production of methyl isonicotinate N-oxide 13.9 g of isonicotinic acid N-oxide was added to 209 ml of methylene chloride, 29.7 g of 1-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline was further added thereto, and the mixture was stirred under argon atmosphere at room temperature for one hour. 32.1 g of methanol was added to this mixture, which was stirred at room temperature for 17 hours. After the solvent was evaporated under reduced pressure, the residue was subjected to silica gel column chromatography. Chloroform-acetone (3:1) was used as an eluent to yield 11.1 g of a white powder. 1H-NMR (CDCl3) δppm: 3.95 (3H, s), 7.88 (2H, d, J=7.25 Hz), 8.22 (2H, J=7.25 Hz) 2) Production of Methyl 2-cyanoisonicotinate 11.1 g of the crystal obtained in 1) was dissolved in 170 ml of acetonitrile, 14.6 g of triethylamine and 21.5 g of trimethylsilylnitrile were added thereto, and the mixture was refluxed under argon atmosphere for 16 hours. After the solvent was evaporated under reduced pressure, the residue was subjected to silica gel column chromatography. Chloroform-acetone (95:5) was used as an eluent to yield 8.44 g of a pale yellow powder. 1H-NMR (CDCl3) δppm: 4.01 (3H, s), 8.08 (1H, d, J=5.45 Hz), 8.24 (1H, s), 8.90 (1H, d, J=5.45 Hz) 3) Production of 2-cyanoisonicotinic acid hydrazide 8.44 g of the crystal obtained in 2) was added to 85 ml of methanol, 1.84 g of hydrazine was further added thereto, and the mixture was stirred under argon temperature for 2 hours. After the solvent was evaporated under reduced pressure, chloroform was added to the residue, which was stirred at room temperature for one hour. The precipitated crystal was filtered, washed with chloroform and dried with a vacuum pump to yield 4.15 g of a pale yellow powder. 1H-NMR (DMSO-d6) δppm: 4.72 (2H, s), 8.05 (1H, d, J=5.12 Hz), 8.31 (1H, s),8.90 (1H, d, J=5.12 Hz), 10.23 (1H, s) 4) Production of the Object Compound 2.67 g of 4-cyanopyridine was dissolved in 40 ml of methanol, 0.83 g of sodium methoxide was added thereto, and the mixture was stirred at room temperature for one hour. Then 4.15 g of the crystal obtained in 3) was added and the mixture was refluxed for 37 hours. After the reaction completed, the precipitated solid was filtered, washed with methanol and dried with a vacuum pump to yield 3.66 g of the object compound as a yellow powder. 1H-NMR (DMSO-d6) δppm: 8.01 (2H, dd, J=4.54, 1.57 Hz), 8.31 (1H, dd, J=5.11, 1.65 Hz), 8.53 (1H, dd, J=1.65, 0.50 Hz), 8.80 (2H, dd, J=4.54, 1.57 Hz), 8.93 (1H, dd, J=5.11, 0.50 Hz) Example 39 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole 1) Production of isonicotinic acid (N-2-tert-butoxycarbonyl)hydrazide-1-oxide 585 ml of methylene chloride was added to 39.0 g of isonicotinic acid N-oxide, and after 34.0 g of triethylamine was further added thereto, the mixture was cooled under argon atmosphere to −15° C. 33.5 g of ethyl chlorocarbonate in 117 ml of methylene chloride was added dropwise to this mixture, which was stirred at a temperature from −5 to −10° C. for one hour. Then 44.4 g of tert-butyl ester of carbamic acid in 117 ml of methylene chloride was added dropwise to this mixture and it was allowed to slowly rise to room temperature while it was stirred. The precipitated solid was filtered after 15 hours, washed with methylene chloride, and dried with a vacuum pump to yield 49.7 g of white crystal. 1H-NMR (DMSO-d6) δppm: 1.42 (9H, s), 7.82 (2H, d, J=7.09 Hz), 8.33 (2H, d, J=7.09 Hz), 9.02 (1H, s), 10.44 (1H, s) Production of 2-cyanoisonicotinic acid hydrazine 1½ P-Toluenesulfonic acid salt 228 ml of dioxane was added to 30.4 g of the crystal obtained in 1), and after 13.1 g of trimethylsilyl cyanide and 38.8 g of N,N-dimethylcarbamoyl chloride were further added thereto, the mixture was stirred under argon atmosphere at 60° C. for 5 hours. After the solvent was evaporated under reduced pressure, the residue was dissolved in ethyl acetate and subsequently washed with 1.5 M sodium carbonate aqueous solution and a saturated saline solution and dried over magnesium sulfate. After the magnesium sulfate was filtered off, the solvent was evaporated under reduced pressure. Ethyl acetate was added to the residue, 68.5 g of p-toluenesulfonic acid monohydrate was added thereto, and the mixture was stirred at room temperature for 22 hours. The precipitated crystal was filtered, washed with ethyl acetate, and dried with a vacuum pump to yield 40.3 g of white crystal 2). 1H-NMR (DMSO-d6) δppm: 2.28 (4.5H, s), 7.12 (3H, dd, J=7.92 & 0.66 Hz), 7.48 (3H, dd, J=7.92 & 0.66 Hz), 8.10 (1H, dd, J=5.11 & 1.81 Hz), 8.39 (1H, dd, J=1.81 & 0.33 Hz), 8.99 (1H, dd, J=5.11 & 0.33 Hz) 3) Production of 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole 9.98 g of 4-cyanopyridine was dissolved in 250 ml of methanol, and after 7.77 g of sodium methoxide was added thereto, the mixture was stirred at room temperature for one hour. Then 40.3 g of the crystal obtained in 2) was added and the mixture was refluxed for 24 hours. After the reaction completed, the precipitated crystal was filtered, washed with methanol, and dried with a vacuum pump to yield 16.3 g of yellow crystal. 1H-NMR (DMSO-d6) δppm: 8.01 (2H, dd, J=4.54 & 1.57 Hz), 8.31 (1H, dd, J=5.11 & 1.65 Hz), 8.53 (1H, dd, J=1.65 & 0.50 Hz), 8.80 (2H, dd, J=4.54 & 1.57 Hz), 8.93 (1H, dd, J=5.11 & 0.50 Hz) 4) Production of 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole 45 ml of ethanol and 15 ml of 1-methyl-2-pyrrolidone were added to 3.0 g of the crystal obtained in 3), and the mixture was heated and stirred at 80° C. for 19 hours. The crystal was filtered, subsequently washed with a mixture of ethanol and 1-methyl-2-pyrrolidone (3:1) and ethanol, and dried with a vacuum pump to yield 2.71 g of yellow crystal. 5) Production of 5-(2-cyano-4-pyridyl)-3-(4-pyridyl)-1,2,4-triazole p-toluenesulfonic acid salt 5 ml of ethanol and 30 ml of water were added to 2.48 g of the crystal obtained in 4), and after 3.8 g of p-toluenesulfonic acid monohydrate was further added thereto, the mixture was stirred at room temperature for 5 hours. The precipitated crystal was filtered, subsequently washed with a mixture of ethanol and water (1:6), water and then ethanol, and dried with a vacuum pump to yield 3.5 g of white crystal. 1H-NMR (DMSO-d6) δppm: 2.28 (3H, s), 7.12 (2H, dd, J=7.75 & 0.50 Hz), 7.48 (2H, dd, J=7.75 & 0.50 Hz), 8.33 (1H, dd, J=5.12 & 1.65 Hz), 8.45 (2H, d, J=6.11 Hz), 8.57 (1H, dd, J=1.65 & 0.66 Hz), 8.96˜9.02 (3H, m) 6) Production of the object compound 17 ml of ethanol and 17 ml of water were added to 3.36 g of the crystal obtained in 5), and the mixture was stirred at room temperature for 30 minutes. A solution of sodium carbonate (0.74 g of sodium carbonate in 17 ml of water) was further added, and the mixture was stirred at room temperature for 2 hours. The precipitated crystal was filtered, subsequently washed with water and ethanol, and dried with a vacuum pump to yield 1.89 g of the object compound as a pale yellow crystal. SYNTHESIS WO2014017516A1  (First step) The first step, 4 - is a step of obtaining a compound (3) is reacted in the presence of an alkali metal alkoxide, cyano-N-oxide and (2), and isonicotinic acid hydrazide. 4 used in this reaction - isonicotinic acid hydrazide and (2) a cyano-N-oxide is a known compound both, I can be prepared by known means. The alkali metal alkoxide is used, 6 alkoxide alkali metal C 1-C are preferred, sodium methylate, sodium ethylate and the like can be given as specific examples. The reaction is preferably carried out in a solvent, as the solvent, alcohol solvents such as methanol, ethanol and the like are preferable. The reaction is preferably first in a solvent, is treated with an alkali metal alkoxide compound (2) and then to react the isonicotinic acid hydrazide. First, heated to reflux under cooling, at 80 ℃ from 15 ℃ preferably, 30 minutes and 12 hours in general, the reaction temperature in the reaction with an alkali metal alkoxide (2) with the compound is reacted 1-4 hours, preferably about. Under the temperature conditions, using an excess amount or one equivalent of 30 minutes to 12 hours usually, reaction with isonicotinic acid hydrazide Subsequent to reaction for 1 to 5 hours, preferably. Example 1: Synthesis 4 oxide (3) - - - (4 - pyridin-carbonyl) -4 - N "pyridine hydrazide imide -1 was suspended in 40mL of methanol cyanopyridine-N-oxide and (2) 5.00g, sodium was added to methylate 22.4mg, and the mixture was stirred for 2 hours under 40 ℃ nitrogen atmosphere. was cooled to room temperature. reaction solution was stirred for 4 hours at 40 ℃ was added isonicotinic acid hydrazide 5.71g at the same temperature, precipitated The filtrated crystals were, washed with methanol 15mL, and dried 15 hours at 80 ℃, N "- to give (3) 9.60g oxide - (4 - pyridin) -4 - pyridine-hydrazide imide -1. 1 H-NMR (DMSO-d 6) δ (ppm): 6.98 (br, 2H), 7.81 (d, 2H, J = 5.77Hz), 7.85 (d, 2H, J = 7 .09 Hz), 8.29 (d, 2H, J = 7.09Hz), 8.73 (d, 2H, J = 5.77Hz), 10.37 (br, 1H) MS m / z: 256 [M-H] - (Second step) The second step is a step of obtaining compound (4) by cyanation agent cyano compound (3). As the cyanation agent used, trialkyl cyanide alkali metal cyanide, sodium cyanide, potassium cyanide and the like, zinc cyanide, trimethylsilyl cyanide and the like. The cyanation reaction is preferably, for example, be carried out (Heterocycles, Vol.22, No.5, 1994) by Reissert Henze reaction. This reaction, for example, to give compound (4) by an organic solvent in the compound (3), and after activation with carbamoyl halide, and reacting the cyano agent. The alkylcarbamoyl halide used in the carbamoylation is a first step in Reissert Henze reaction, 6 alkylcarbamoyl halide di C 1-C dimethylcarbamoyl chloride, and di-propyl carbamoyl chloride can be used, preferably, dimethylcarbamoyl is chloride. The solvent used in this reaction, N, N-dimethylformamide, N, N-dimethylacetamide, N-methylpyrrolidone, tetrahydrofuran and acetonitrile can be used, however, N, N-dimethylformamide is preferred. Further, 15 ~ 60 ℃, more preferably 30 ~ 50 ℃ reaction temperature. The reaction time is preferably 1 to 24 hours, more preferably 1 to 3 hours. As the cyanation agent used in the cyanation reaction followed, cyano agents above can be used, sodium cyanide, potassium cyanide, zinc cyanide, and trimethylsilyl cyanide, and more preferably, it is sodium cyanide . -20 ~ 60 ℃ is preferred, more preferably -10 ~ 40 ℃, reaction temperature is 1-4 hours. Is a novel compound (4) The compound obtained in this second step, it is useful as an intermediate for the production of compound (1). If through Compound (4) can be synthesized in good yield and easily without the need for purification in the second step is also possible, and can be produced (1) Compound industrially efficiently compound (4). Synthetic N "hydrazide (4) - (4 - pyridine carbonyl) -4 - pyridine carboxylic acid N'-(carboxylic imidoyloxy - 2 - - cyano-4) Example 2 4 pyridine hydrazide imide -1 - oxide ( was suspended in N, N-dimethylformamide 48mL and 3) 10.0g, under nitrogen atmosphere, followed by stirring for 1 hour was added dimethylcarbamoyl chloride 9.20g at 40 ℃. was added sodium cyanide 2.48g at the same temperature, After cooling to 5 ℃ below. reaction mixture was stirred for 1 hour, the crystals were collected by filtration. precipitate was successively added dropwise a 5% aqueous sodium bicarbonate solution 100mL, and 100mL water, and washed with water 100mL, at 80 ℃ for 15 h and dried under reduced pressure to give 4 - hydrazide (4) 9.28g of pyridine-carboxylic acid N'-(carboxylic imide yl - 2 - cyano-4). 1 H-NMR (DMSO-d 6) δ (ppm): 7.15 (br, 2H), 7.82 (d, 2H, J = 5.61Hz), 8.14 (d, 1H, J = 5 .11 Hz), 8.37 (s, 1H), 8.75 (d, 2H, J = 5.61Hz), 8.86 (d, 1H, J = 5.11Hz), 10.47 (br, 1H ) MS m / z: 265 [M-H] -  (Third step) The third step is a step of obtaining a compound (1) by the presence of an acid catalyst, the cyclization reaction of the compound (4). As the acid, organic phosphoric acid, p-toluenesulfonic acid, such as hydrochloric acid, inorganic acids can be used, inorganic acids, phosphoric acid is particularly preferable. As the reaction solvent, water, 2 - butanol, 2 - mixed solvent of alcohol and water or alcohol, propanol, ethanol and the like can be used, but water and 2 - I was mixed 5:1 to 10:1 butanol solvent. The reaction temperature and time, 60 ~ 100 ℃, preferably 2 to 12 hours at 70 ~ 90 ℃, I want to 8-10 hours, preferably. Intermediates and compounds of the present invention the method (1) can be isolated and purified from the washed reaction mixture, recrystallization, by means of various conventional chromatography. Example 3: 4 - [5 - (pyridin-4 - yl)-1H-1, 2,4 - triazol-3 - yl] pyridine-2 - carbonitrile 4 Synthesis of (1) - pyridine-carboxylic acid N'- (2 - cyano-4 - carboxylic imide yl) water 82mL, 2 hydrazide (4) 9.25g - butanol was added 8.2mL, phosphate 4.00g, was stirred for 8 h at 80 ℃. After cooling to room temperature, the reaction mixture was precipitated crystals were collected by filtration, water: 2 - were washed with a mixed solution of 92.5mL butanol = 10:1. The 13 h and dried under reduced pressure at 80 ℃ crystals obtained 4 - [5 - (pyridin-4 - yl) - 1 H-1, 2,4 - triazol-3 - yl] pyridine-2 - carbonitrile (1 I got a) 7.89g. Topiroxostat 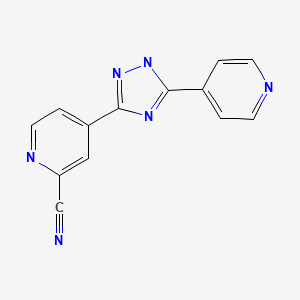 1 H-NMR (DMSO-d 6) δ (ppm): 8.02 (dd, 2H, J = 4.59,1.62 Hz), 8.32 (dd, 1H, J = 5.13,1. 62Hz), 8.55 (dd, 1H, J = 1.62,1.08 Hz), 8.80 (dd, 2H, J = 4.59,1.62 Hz), 8.93 (dd, 1H, 5 .13,1.08 Hz) MS m / z: 247 [M-H] - PATENT WO2014017515A1 Synthetic water-carbonitrile p-toluenesulfonate - pyridine Example 1: 4 - [yl 5 - (pyridin-4 - yl)-1H-1, 2,4 - - -3 triazol]: 2 - butanol = was added monohydrate 6.62g p-toluenesulfonic acid in a mixed solution of 55mL of 10:1, 4 at 80 ℃ - [5 - (pyridin-4 - yl)-1H-1, 2,4 - yl] pyridine-2 - - triazol-3 was added carbonitrile 7.85g, and the mixture was stirred at the same temperature for 1 hour. After cooling to room temperature, the reaction mixture, and the precipitated crystals were collected by filtration, and water: 2 - were washed with a mixed solution of 40mL of butanol = 10:1. The dried under reduced pressure for 10 hours at 80 ℃ crystals obtained 4 - [5 - (pyridin-4 - yl)-1H-1, 2,4 - triazol-3 - yl] pyridine-2 - carbonitrile p-toluene I got a sulfonate 12.6g. 1 H-NMR (DMSO-d 6) δ (ppm): 2.29 (s, 3H), 7.11 (m, 2H), 7.48 (dd, 2H, J = 6.48,1.62 Hz ) ,8.32-8 .35 (m, 3H), 8.57 (dd, 1H, J = 1.62,0.81 Hz) ,8.94-8 .98 (m, 3H) - [5 - (pyridin-4 - yl)-1H-1, 2,4 - triazole and potassium carbonate 8.22g, 4 in a mixed solution of 80mL of ethanol = 9:1: preparation water of crystal form I: Example 2 I was dissolved carbonitrile p-toluenesulfonate 10.0g - -3 - yl] pyridine-2. After stirring for 5 hours plus 15mL 6M hydrochloric acid at 20 ℃, was the precipitated crystals were collected by filtration, and washed with water 100mL. The 23 h and dried under reduced pressure at 80 ℃, 4 - to obtain carbonitrile 5.78g - pyridin-2 [yl 5 - (pyridin-4 - yl)-1H-1, 2,4 - - -3 triazole. Having a DSC as shown in FIG 4 and the powder X-ray diffraction pattern shown in FIG 1, the resulting crystals were type-I crystals. 1 H-NMR (DMSO-d 6) δ (ppm): 8.02 (dd, 2H, J = 4.59,1.62 Hz), 8.32 (dd, 1H, J = 5.13,1. 62Hz), 8.55 (dd, 1H, J = 1.62,1.08 Hz), 8.80 (dd, 2H, J = 4.59,1.62 Hz), 8.93 (dd, 1H, 5 .13,1.08 Hz) Melting point: 327 ℃ N, N carbonitrile 40.0g - preparation of 4 Form II - [5 - (pyridin-4 - yl)-1H-1, 2,4 - yl - triazol-3]-2: Example 3 - dimethylformamide was added 300mL, and stirred for 25 min at 150 ℃. After cooling to room temperature the solution, and the precipitated crystals were collected by filtration, and washed twice with water 200mL, 4 and dried under reduced pressure overnight at 80 ℃ the crystal - [5 - (pyridin-4 - yl)-1H-1 , 2,4 - I got carbonitrile 30.4g - yl] pyridine-2 - triazole-3. Having a DSC as shown in FIG 5 and powder X-ray diffraction pattern shown in FIG 2, the resulting crystals were type II crystals. 1 H-NMR (DMSO-d 6) δ (ppm): 8.02 (dd, 2H, J = 4.59,1.62 Hz), 8.32 (dd, 1H, J = 5.13,1. 62Hz), 8.55 (dd, 1H, J = 1.62,1.08 Hz), 8.80 (dd, 2H, J = 4.59,1.62 Hz), 8.93 (dd, 1H, 5 .13,1.08 Hz) Melting point: 327 ℃ The 25 ℃, about 2g carbonitrile, - preparation of the hydrate 4 - [5 - (pyridin-4 - yl)-1H-1, 2,4 - triazol-3 - yl] pyridine-2: Example 4 I was stored for 14 days under conditions of relative humidity 97%. Having a DSC as shown in FIG 7 and the powder X-ray diffraction pattern shown in FIG 3, the obtained crystal was a hydrate. 1 H-NMR (DMSO-d 6) δ (ppm): 8.02 (dd, 2H, J = 4.59,1.62 Hz), 8.32 (dd, 1H, J = 5.13,1. 62Hz), 8.55 (dd, 1H, J = 1.62,1.08 Hz), 8.80 (dd, 2H, J = 4.59,1.62 Hz), 8.93 (dd, 1H, 5 .13,1.08 Hz) Melting point: 327 ℃ Test Example: solubility test Type I crystal by crystal form, II-type crystal, and water solubility of the hydrate was calculated by absorbance measurement method, a saturated solution concentration of each sample. I Figure 8 shows the results.Whereas the 6.2μg/mL water solubility of crystalline Form I, II type crystal 4.2μg/mL, hydrate was 1.9μg/mL. From Figure 8, the water solubility of Form II and Form I crystals is good, water-soluble type I crystal is particularly good. NMR BMCL Volume 19, Issue 21, 1 November 2009, Pages 6225–6229 http://www.sciencedirect.com/science/article/pii/S0960894X09012372?np=y view compd 39 and ignore rest  TOPIROXOSTAT, FYX O51 TOPIROXOSTAT, FYX O51view compd 39 and ignore rest SUPP INFO.......https://docs.google.com/viewer?url=http://www.sciencedirect.com/science/MiamiMultiMediaURL/1-s2.0-S0960894X09012372/1-s2.0-S0960894X09012372-mmc1.doc/271398/FULL/S0960894X09012372/50d911fe734c16dfb94912d481cb466a/mmc1.doc
Inoue, Tsutomu; Sato, Takahiro; Ashizawa, Naoki; Iwanaga, Takashi; Matsumoto, Koji; Nagata, Osamu; Nakamura, Hiroshi Bioorganic and Medicinal Chemistry Letters, 2009 , vol. 19, 21 pg. 6225 - 6229 WO 2012060308 WO 2007148835 WO 2005009991
C1=CN=CC=C1C2=NC(=NN2)C3=CC(=NC=C3)C#N 9 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


























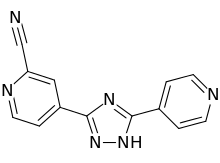
Thank you for this post. This is very interesting information for me. Who can do my assignment for me ?
ReplyDeleteDrug Synthesis International: Ostat Series >>>>> Download Now
Delete>>>>> Download Full
Drug Synthesis International: Ostat Series >>>>> Download LINK
>>>>> Download Now
Drug Synthesis International: Ostat Series >>>>> Download Full
>>>>> Download LINK mf
Allassignmenthelp.com is the top assignment help provider in the US, the UK and Australia. With more than 100 thousand assignments delivered in the last few years, we are the top choice to buy Assignment help.
ReplyDeleteOnline Assignment help
Amazing job. These people are professional, prompt, courteous, and forgiving...because the first time I tried to get their help I was so upset from getting ripped off by course hero and chegg tutors that I flipped out on these guys and blew it. Came to them for help for my girl a month later and they hooked her up...I'll be very surprised if she does not pull an A on this assignment. And when she's happy I am too :) Also they could have taken advantage of me pricewise and they didn't. Outstanding.For more detail: Assignmnet help
ReplyDeleteThanks for the well-written post and I will follow your updates regularly.
ReplyDeleteMachine Learning course in Chennai
Machine Learning Training in Chennai
RPA Training in Chennai
RPA course in Chennai
Blue Prism Training in Chennai
Blue Prism Training Chennai
Automation Anywhere Training in Chennai
UiPath Training in Chennai
Everybody is colossal cognizant going to put a convincing picture before their educator. For achieving this desire progressively activity, you neither miss the obligation regarding course consummation inside committed time or learning propensity attribute.
ReplyDeleteHuman Resource Homework Help
Psychology Homework Help
thankes to Dr oniha, who cured me of fallopian tube blockage and fibroid. i married for over eight years wthout a child of my own. because of the problem with my fallopian tubbe and fibroid, until i came in conntact with Dr oniha's testimony and recommendations online, on how he has cured and healed so many people of the same illment, and i decided to contact him, and he administered his medicatons on me, and i became pregnant in two monnths. incasse you wannt to reach him for a similar problem, yoou can call or whatsap him on +2347089275769 or email: dronihaspell@yahoo.com
DeleteAssignment experts
ReplyDeleteAssignment help expert
Assignment Help Service
Assignment Help Services
Assignment assistance
Assignment Help Company
Online Assignment
Online Assignment Solution
You are doing a great job. I would like to appreciate your work for good accuracy
ReplyDeleteRegards,
Salesforce Training | Online Course | Certification in chennai | Salesforce Training | Online Course | Certification in bangalore | Salesforce Training | Online Course | Certification in hyderabad | Salesforce Training | Online Course | Certification in pune
We are a world-class certified, successful, and self-governing third party QuickBooks support provider, which is providing the reliable and unlimited QuickBooks support services in very affordable charges. If you want to set up QuickBooks online, you can take full and specialized guidance from experienced QuickBooks experts to set up QuickBooks online successfully. Having many years of experience, our certified QuickBooks professionals are very experienced to set up QuickBooks in the proper ways. If you are facing any problems with Setup QuickBooks Online, our certified QuickBooks experts are very trained to guide you properly for any type of accounting troubles. Our certified QuickBooks experts are sitting online to provide quick help for any minor or major errors related to QuickBooks.
ReplyDeleteWow Good Post
ReplyDeleteCommercial Law assignment help,
Commercial Law assignment service,
Commercial Law assignment expert,
Amazing Post Keep it up
ReplyDeleteLaw Assignment help ,
Online Law assignment help ,
Fake Bank Statement
ReplyDeleteFake Bank Statement
Fake Bank Statement
Fake Bank Statement
Fake Bank Statement
Fake Bank Statement
Fake Bank Statement
Fake Bank Statement
Our highly experienced Garmin GPS technical team can assist you in downloading and installing the latest maps with simple steps. If you cannot access Garmin Express website, then you can use Garmin map updater as an alternative to updating maps. However, you will need to follow some instructions while using the updater. You must remove the SD card from your GPS device during the process and choose the Mass Storage Mode when GPS connects to your PC. If you are using it for another time, then the procedure starts automatically. While for the first time, you need to follow the on-screen instructions to complete the process. You can also check the version of the map from Settings, click Map & Vehicle and then choose my Maps. If you need any clarification or have any query, then feel free to ask.
ReplyDeleteAs a result, you lose your marks without doing any mistakes. So, connect with Assignment Helpers, tell them what you want or your queries and let them guide you on finishing your assignment smoothly.
ReplyDeleteThe HP printer not printing issue generally occurs when you are using the corrupted or missing printer driver. But firstly, you must check whether your printer is not printing a particular color or not printing at all! If you are facing issues with a specific color, that means either the ink cartridge is empty, dried up, or expired, and in that case, you need to replace it with genuine HP ink cartridges. And fixing the printer not printing at all problems, you will have to uninstall the printer driver from your computer and then reinstall it from the HP support website. After the complete procedure, restart your PC to save the settings and get back your printer to running state.
ReplyDeleteLearning is a hard process if not handled with proper tools. We offer Test Banks and Solution Manuals for the most popular textbooks including Leading And Managing In Canadian Nursing Canadian Edition Test Bank. View today!
ReplyDeleteTally ERP 9 Activator can be utilized in distinct methods are: consumers shell out the lender and other charges out of your home and just about anywhere, visualize if one does your organization competence personally. Its unit installation is rather easy and clear-cut. Tally ERP 9 Key is usually an entire product which draws in its exclusive effortlessly,
ReplyDeleteQuickBooks latest release Desktop can be either updated manually or automatically. If automatic updates are turned on, then it will be updated itself once and when the update is available. But when you have selected manual updates, you will have to update it yourself and check for the updates yourself once in a month.
ReplyDeleteThe timestamp disappointment can be a reason for concern and can prompt Cash App refund disappointment. Along these lines, you can utilize the strategies that are accessible in the investigating locales or you can utilize the help that is accessible in the assistance community. Notwithstanding that, the tech vids can be an extraordinary shelter with regards to fixing a mistake.
ReplyDeleteThis is a comprehensive and interesting article. You can enter the chemical analysis department of the drug control department.
ReplyDeleteI want to advise the cool best payout online slots in Canada.
It's all about thousands of new interesting slots. Here is any thematic of it. Best way to try your luck and earn some cash. Try it on this website: https://keencounters.com/
The article is very nice, “thank” you for sharing it! ?
ReplyDeleteFinePrint Crack
Getting top grades in college Culinary Hospitality Travel Tourism can be quiet the challenge if you do not have the right study resources. Be it exams or homework, Online Culinary Hospitality Travel Tourism Test Banks and Solution Manuals from GrowMyGrade can come to your aid to help you out of tight spots.
ReplyDeleteCyberGhost VPN V7 Premium Crack is the best online program that provides full access to you all over the internet. It lets you get rid of the contents that are block or somehow restricted. With it, you will be able to enjoy the blocked websites and other access that are possessed by the country or by the IT authority. Similarly, it is providing smooth internet access by nonstop no interruption in playing games..
ReplyDeleteReally, this article is truly one of the best, information shared was valuable and resourceful Very good work thank you.
ReplyDeletetally training in chennai
hadoop training in chennai
sap training in chennai
oracle training in chennai
angular js
training in chennai
Thanks for giving me grateful information. I think this is very important to me. Your post is quite different. That's why I regularly visit your site.
ReplyDeleteAheck Also This - SSC CHSL 10+2 Recruitment
The best method to approach a study session is through the best learning tools available online. GrowMyGrade is one of the best online sellers for testbanks and solution manuals like Test Bank For International Accounting 4th Edition.
ReplyDeleteGreat, submit, very informative.
ReplyDeleteI ponder why the opposite experts of this sector don’t realize this.
You must proceed your writing.
I’m confident, you have
a great readers’ base already!
tipard video converter crack
flvto youtube downloader crack
4k video downloader crack
iobit uninstaller procrack
This is a wonderful article, Given so much info in it, These type of articles keeps the users interest in the website, and keep on sharing more ... good luckText irql not less or equal
ReplyDeleteThis site helps to clear your all query.Hpu ba 3rd Year Result 2020 Check Latest Updates about your Board and Universities Timetable, Admit Card and Results. We have all universities and board data to provide latest news. All Indian Board & University Exam Timetable-Result.com BA 3rd Year Exam Routine 2021
ReplyDeleteNot able to print and get Epson Printer Offline messages on your screen? Well, as the message describes, your Epson printer Offline fix and hence cannot print anything. Such an error can occur due to various reasons like if your printer isn’t configured properly, if there’s some issue with the connection, or if you have enabled the Use printer offline feature accidentally. Depending on the issue, you can work on the fix accordingly. In the beginning, you should try restarting your printer, computer, and WiFi (in case of a wireless printer) and check if that works for you. Secondly, ensure all the connections are made properly and fix any loose ends. Also, check your printer screen, for any error codes and work on that first.
ReplyDeleteYou can use our Epson printer offline troubleshooting guide for a complete solution regarding your issue. Or you can also reach us directly on our Epson Printer Support number for remote assistance from our printer experts.
Your post appears in a variety of dynamic ways that assure us that there are still bloggers like you who can write on any topic in the best possible way. Thanks for this outstanding share here. Buy Essay Online
ReplyDeletejava homework help though this forum is not very big, it's still quite useful. Your questions may not be answered right away. This forum is ideal for Java homework Help who want to help fellow programmers, while simultaneously building their Java knowledge along the way.
ReplyDeleteAvid Pro Tools Key lets you work in a very smooth way that all tools are functioning under your fingers. Do whatever you want like mix audio to MAC and windows. Additionally, you can utilize its complete editing tools that are more than any MIDI technologypro tools 12 crack mac.
ReplyDeletecanon printers have become the most selling printer everywhere on the globe yet in some cases, user face loads of issues, for example, Canon Printer in Error State Windows 10 or other error messages. Innovation is developing step by step and another hand, ordinance printer is improving their designs and updating the model quickly.
ReplyDeletewhy does my canon printer say in error state
All those students who want instant help for writing their assignments can get in touch with our academic writers at any time. Our expert team is working in this field for many years and has knowledge of each subject properly. Thus, share your assignment topic online and be carefree. Via our Online Homework Help services, you can assistance of our Homework Helper and submit your project requirements easily. Our experts will quickly work on it and revert you before your due date of submission. Make sure you give the essential requirements and details of your projects precisely. The service is accessible 24*7. Therefore, there is no restriction of time; you can get in touch anytime and hire our homework writers from anywhere.
ReplyDeleteBest Assignment Experts is such an academic writing help service provider who has the knowledge and experience in providing Assignment writing help services to students. You can take their services at the most affordable pricing with assured timely delivery of papers.
ReplyDeleteTherefore, you don’t need to worry about missing deadlines and other factors for your project submission. Browse the website of a Write My Essay provider for more information.
ReplyDeleteMYOB perdisco assignment help is for students so they can learn the art of taxation and accounting solutions. It helps them to learn the concepts of these subjects and become better once they start working for a company. Perdisco myob assignment help is provided by some of the top accounting and taxation experts who are always ready to help students and provide the best services at affordable prices as well.
ReplyDeleteI saw so many testimonies about Dr Itua a great HERBAL DOCTOR that can cure all kind of diseases and give you the rightful health to live a joyful life, I didn't believe it at first, but as the pain got worse and my life was at risk after visiting my therapist numerous times for combination of treatments. And no changes so I decided to take a try, I contacted him also and told him I want a cure for Vulvar cancer/ Testicular cancer and it was Stage IIIA, he gave me advice on what I must do and he delivered it to me in my state which I use according to his instruction, and today I must say I am so grateful to this man Dr Itua for curing me from Vulvar cancer/ Testicular cancer and for restoring me back to my normal health and a sound life, I am making this known to every one out there who have been living with cancers all his life or any sick person should not waste more time just contact him with his details below- WhatsApp- +2348149277967 Email drituaherbalcenter@gmail.com, believe me this man is a good man with Godly heart, this is the real secret we all have been searching for. Do not waste more time contacting him today for you to also live a sound and happy life. He cures the following disease, thyroid Cancer, Uterine cancer, Fibroid, Arthritis, Brain Tumor,Fibromyalgia, Bladder cancer, Brain cancer, HIV, Herpes, Esophageal cancer, Gallbladder cancer, Gestational trophoblastic disease, Head and neck cancer, Hodgkin lymphoma Intestinal cancer, Kidney cancer,Hpv, Lung cancer, Melanoma,Mesothelioma, Multiple myeloma,Neuroendocrine tumors Non-Hodgkin lymphoma, Oral cancer, Ovarian cancer, Sinus cancer, Hepatitis A, B/C, Skin cancer, Soft tissue sarcoma, Stroke, Lupus, Spinal cancer, Stomach cancer, Vaginal cancer,Vulvar cancer, Testicular cancer,Tach Diseases, Pancreatic Cancer, Leukemia, Liver cancer, Throat cancer, Alzheimer's disease, Chronic Diarrhea,Copd, Parkinson,Als,Adrenocortical carcinoma Infectious mononucleosis.
ReplyDeleteFacing trouble to open Quicken? Here are the quick steps to eliminate the problem along with you will also learn how to quicken won’t open issue.
ReplyDeleteWoah! I’m really enjoying the template/theme of this website.
ReplyDeleteIt’s simple, yet effective. A lot of times it’s very
difficult to get that “perfect balance” between user friendliness and appearance.
I must say that you’ve done a very good job with this.
Also, the blog loads very quickly for me on Firefox.
Exceptional Blog!
screenpresso pro crack
voicemod pro crack
morphvox pro crack
bulk image downloader crack
radmin vpn crack
adobe animate cc crack
If you recently purchased a new Epson printer, you can encounter some technical difficulties, such as how to troubleshoot an Epson printer. Perhaps you can't configure the printer at the outset. Other things could trigger a working hurdle, too. Still, because we are here to help you to face the problems, we have nothing to think about. If you cannot print in black and colored format, you cannot bind to the device or run a problem with the drivers; what you need to do is contact us. Epson inkjet printers are eco-friendly printers that deliver outstanding quality printing with good speed and reliability. Epson has two variants of the printer with different functionality such as Laser Printer and Inkjet Printer. Both the type of printers has their strength and quality. So with the help of this wireless printer, you can print from the device easily. Let’s see how to do the Epson Connect printer setup.
ReplyDeletePlease Visit our site: Epson Printer Setup Utility
Nicely written & done.
ReplyDeleteI started writing in the last few days and realized that lot of
writers simply rework old ideas but add very little of value.
It’s fantastic to read an informative post of some genuine value to myself and your other followers.
It is on the list of things I need to replicate as a new blogger.
Reader engagement and content quality are king.
Some terrific ideas; you’ve most certainly managed to get on my list of blogs to watch!
Carry on the terrific work!
Well done,
Cheryl.
Thank you!
ik multimedia amplitube crack
Virtual events put event stakeholders in the unfamiliar position of having to convey that value virtually. event marketing and event registration tools
ReplyDeleteThat is a great tip especially to those new to the blogosphere.
ReplyDeleteBA Examination Date Sheets
Best Outdoor Umbrella For High Winds will provide you sunlight light and weather protection: This umbrella regularly mounted outdoors the home in garden locale you’re able to also use on inns at roof very best for batter ambiance. That is extensive term product when you consider that it designed with polyester fabric and metal done stand. The movable metal stand gives you correct sun light protection. It's possible to also retain rest outside your home with pals and family for those who have this. The made material doesn’t make it possible for and heat and rays under the shade of umbrella.
ReplyDeleteWe are the best Female Escorts Service in Thane. We provide the super hot figure and looking gorgeous Call Girls in Thane. Our Call Girls are really so good at behavior and fully cooperated with her clients. They can fulfill burning physical satisfaction and do super unlimited sex. My Name Is Dipti Kaur and I am Cute Sexy And High Educated Independent Girl. I Work For Female Escorts in Thane. I work as Call Girl in Thane Outcall Or Incall. I Know About my Client What's Expected From me. I am Dipti your dream girl with a beautiful face with a height of 5'6 ft. I have the perfect body figure of 34-28-32 with a fair complexion, a well-maintained figure with attractive butts. I will satisfy you wholly from head to toe. You will enjoy each and every moment with me. I am always eager to meet new people. So book me soon for taking immense pleasure and fun with me.
ReplyDeleteoutdoor wood sauna
ReplyDeleteWAJA sauna is specialist manufacturer of top quality sauna products. Products include sauna rooms, steam rooms, barrel saunas, wooden hot tubs, and all kinds of sauna accessories.
I feel that is among the so much important information for me. And i am satisfied reading your article. However should statement on few common issues, The site style is great, the articles is in point of fact great : D. Excellent process, cheers.
ReplyDeletedvdfab 11 crack
inpixio photo focus pro crack
lightworks pro crack
roxio toast titanium crack
lucky patcher apk cracked
iobit malware fighter crack
morphvox pro crack
sony vegas pro patch setup free crack
driver checker crack
MYOB help Australia is cheap and affordable for every student. We conduct monthly evaluation tests and help students with assignment works. MYOB Help Australia
ReplyDeletevery interesting blog places check my site pest control london thanks pest control enfield
ReplyDeletepest control lewisham
Winzip Crack Code is truly a extremely good archive software. It can be a good way to operate with compressed files. It presents you extensive obtain towards extracting file in any format. WINZIP is gold stander software which allows you to regulate, shield, share and edit your archive files. This tool supports varying varieties of archives structure together with 7 zip, gzip, tar, Xz, z, cab, Rar, and QZ. WinZip also a decent file manager that compressed the file and enable it to be secured. It will allow the user to a whole lot more security alternative by which consumer non-public file defend
ReplyDeleteTires/Wheels Sales and Service
ReplyDeleteWow Tire and Auto Repair have the equipment and technicians to accurately diagnose and repair all tire related problems. We offer the widest selection of tires and our team has years of experience of repairing and selling tires. We provide prompt and quality service at a great cost. Visit our location to see our selection of tires. We have manufacturers tire rebates available, inquire within.
Tires Wheels Sales and Service in Calgary
Lightworks Pro 14.6 Full Crack
ReplyDeleteAndroid Apks - is a place where you can fiAndroid Apks - is a place where you can find paid best android apk apps games to download free full version for mobile, tablets.
ReplyDeletetango app for android free download apk
According to a new report by Expert Market Research, ‘Automotive Turbocharger Hose Market Size, Share, Price, Trends, Growth, Report and Forecast 2021-2026’, the Automotive Turbocharger Hose Market size was valued at USD XX million in 2020 and is predicted to register a CAGR of XX% from 2021 to 2026.
ReplyDeleteDownload a free Sample Report : Automotive Turbocharger Hose Market Size, Report & Forecast
Well written informative post. You have mentioned such valuable information for beginners. It helps them lot. Good work, keep it up. by pes file converter
ReplyDeletewe have a lot of top notch PhD designing assignment help Australia specialists who are intensive with every single field of designing. Be it a compound, mechanical, gadgets, computer science or some other, as a solid designing assignment master, we attempt to never let any of your inquiries perplexing. assignment provider
ReplyDeleteassignment help melbourne
Call Girls In Lepakshi !!
ReplyDeleteCall Girls In Kozhikode !!
Call Girls In Koyilandy !!
Call Girls In Kovalam !!
Call Girls In Kottayam !!
Call Girls In Koramangala !!
Call Girls In Kollam !!
Call Girls In Kodungallur !!
Call Girls In Kasaragod !!
Call Girls In Kannur !!
Call Girls In Kanhangad !!
Call Girls In Kalamassery !!
Call Girls In Indira Nagar !!
Call Girls In Horsley Hills !!
Call Girls In Hampi !!
Call Girls In Guruvayur !!
Call Girls In Ernakulam !!
Call Girls In Electronic City !!
Call Girls In Chilakaluripet !!
Call Girls In Bheemunipatnam !!
Call Girls In Bangalore !!
Call Girls In Banaswadi !!
Call Girls In Anakapalle !!
Call Girls In Amravati !!
Call Girls In Amalapuram !!
Call Girls In Aluva !!
Call Girls In Alappuzha !!
Call Girls In Kochi !!
Call Girls In Malappuram !!
Call Girls In Manjeri !!
You have done a great job on this article. It’s very readable and highly intelligent.
ReplyDeleteYou have even managed to make it understandable and easy to read. You have some real writing talent. Thank you
amazon quiz
gk quiz
general knowledge quiz
english stories
bedtime stories
bedtime short stories
english short stories
ReplyDeleteAssignment Help in Adelaide.
Best Assignment Experts offers Adelaide custom assignment assistance online. Students at the University of South Australia, Flinders University and Torrens University Australia in Adelaide can get original, unique Assignment Help Adelaide
from the company. Since the inception of the company.
Sankey diagram is a very useful visualization to show the flow of data. There are many tools but I found all of them really hard for creating Sankey Diagram. But ChartExpo provides you a better and easiest way to create the Sankey Diagram in no time without coding only on few clicks. Read more here : Sankey Diagram Excel . Read more articles here : https://businessanddigitalmarketing.blogspot.com/2021/07/how-effective-are-google-ads.html.
ReplyDeleteMost Popular Download Games. Download free full version games for your PC. Minecraft. Let your imagination fly and build your own world.
ReplyDeletenfs hot pursuit download highly compressed
This software is very helpful for as, thanks for sharing Nero Burning Rom Crack
ReplyDeleteAnd Also Visit This Site ProCrackerz
An Omega Personality is one defined outside of the traditional hierarchy of dominance. They have the intention to neither climb to the top nor give way for others to rise above them. They simply revere themselves as outside of this biological system. They will either focus energy on themselves irrelevant of the hierarchy, or focus on an ideal that exists beyond the immediate entities in their vicinity.
ReplyDeleteAVG PC TuneUp Crack Excellent piece of work, and I am in wonder how you manage all of these content and his entry. I would like to say you have superb capabilities related to your work, and lastly, please keep it up because I am looking for the more.
ReplyDeleteAnd Also Visit This Site ProCrackerz
Wonderful blog!
ReplyDeleteHave you any useful suggestions for aspiring writers?
I intend to establish my own site soon, however,
I'm a bit puzzled about it
Would you advise a free platform like WordPress or a paid option?
There are so many alternatives that I am completely puzzled.
Thank you!
Whoa!
serato sample vst
wi fi hacker crack
I am always searching online for articles that can help me. There is obviously a lot to know about this. I think you made some good points in Features also. Keep working, great job ! 바카라사이트
ReplyDeleteWonderful illustrated information. I thank you about that. No doubt it will be very useful for my future projects. Would like to see some other posts on the same subject! 바카라사이트
ReplyDeleteWow, incredible blog format! How lengthy have you been blogging for? you make running a blog glance easy. The full glance of your site is fantastic, as smartly the content material 바카라
ReplyDeleteThis is a really too good post. This article gives truly quality and helpful information. I’m definitely going to look into it. Also, Check Get paid to shop
ReplyDeleteMATLAB Crack is an all in one software for creating and designing high-level programs. With this, you can also integrate many different programming paradigms.
ReplyDeletematlab-crack
This is a very useful and important information, it was so useful to me and other readers, thank you for always feeding the readers with interesting and useful information, your blog is indeed doing very well, kudos, meanwhile you can checkout this UBA bank transfer pin
ReplyDeleteThank you sharing effective informations.The basic function of the HP wireless assistant is to monitor the connections within the networks, which allows you to manage the wireless local area network, wireless wide area network along with Bluetooth wireless devices with one graphical interface. This software displays the status of all the wireless devices and allows you to enable or disable all the wireless devices.
ReplyDeleteBellsouth Email Login Bellsouth email administration is possessed and constrained by a famous American organization. In any case, since it has converged with AT&T, Bellsouth clients can get to their Bellsouth email account utilizing the AT&T email login page.
ReplyDeleteI am very impressed with your post because this post is very beneficial for me and provide a new knowledge…
ReplyDeletefolder lock Crack
backup4all pro crack
mixpad crack
iMindMap Pro crack
Minitab Crack
dvdfab crack
FireShot Pro crack
Crackny.com
Coinbase support number organization's CEO reprimands controller in series of tweets How Coinbase's Public Debut Affects Bitcoin and Crypto YOU MAY ALSO LIKE UP NEXT How Coinbase's Public Debut Affects Bitcoin and Crypto How Coinbase's Public Debut Affects Bitcoin and Crypto The posting of Coinbase, the biggest bitcoin trade in the U.S., acquaints another way with put resources into digital currencies. WSJ clarifies how Coinbase is attempting to remove itself from the dangers of bitcoin to prevail on Wall Street. Contact Coinbase Support Number Photograph delineation: George Downs By Dave Michaels and Paul Vigna Refreshed Sept. 8, 2021 3:25 pm ET PRINT TEXT The Securities and Exchange Commission is exploring Coinbase Global Inc. over a loaning program the organization intends to advertise and has shown it would sue the organization over the contribution, Coinbase said Tuesday. Coinbase fellow benefactor and Chief Executive Brian Armstrong uncovered the debate in a progression of tweets late Tuesday. He called the SEC's activities "questionable" and "terrorizing strategies away from plain view," and said other crypto organizations can offer such projects.
ReplyDeleteI’ve been browsing online greater than 3 hours nowadays, yet I by no means discovered any interesting article like yours.
ReplyDeleteIt’s pretty price sufficient for me. In my opinion, if all
webmasters and bloggers made just right content material as
you did, the web will probably be a lot more useful than ever before.
카지노사이트
wep
My assignment help is the best answer for the individuals who need the best assignment help online. we have very much experienced assignment specialists that offer assignment help anytime. Recruit us today!
ReplyDeletemyassignmenthelp
In view of the popularity— as a well-known email provider and the benefits— like high-speed internet services that come along with Bellsouth email; there are millions of users getting subscribed to the same and creating BellSouth email accounts.So, bellsouth.net email login
ReplyDeleteThe kind and easy-to-understand explanation made it easy to understand difficult topics. Your writing skills are great. I want to learn great writing skills.
ReplyDelete슬롯머신사이트
Excellent information on your blog, thank you for taking the time to share with us. Amazing insight you have on this, It's nice to find a website that details so much information about different artists. 토토사이트
ReplyDelete
ReplyDeleteThis is one of the best website I have seen in a long time thank you so much, thank you for let me share this website to all my friends. 파칭코
Such an amazing and helpful post this is. I really really love it. It’s so good and so awesome. I am just amazed. I hope that you continue to do your work like this in the future also.
ReplyDelete텍사스홀덤사이트
Very nice article and straight to the point. I don’t know if this is truly the best place to ask but do you folks have any idea where to get some professional writers? Thank you. 바카라사이트
ReplyDeleteI seriously liked, I want details about it, since it is rather wonderful., Cheers pertaining to expressing 토토
ReplyDeleteI wanted to thank you for this excellent read!! I definitely loved every little bit of it. I have you bookmarked your site to check out the new stuff you post 룰렛
ReplyDeleteAn M3U file, also known as Moving Picture Experts Group Audio Layer 3 Uniform Resource Locator, is a computer file engineered for a multimedia playlist M3u Playlist Url Free.
ReplyDeleteI think the admin of this web page is actually working hard in support
ReplyDeleteof his web site, for the reason that here every information is quality. 카지노
ReplyDeleteHello mates, nice piece of writing and fastidious arguments
commented at this place, I am really enjoying by these.
Feel free to visit my blog - 일본경마
"Hey Everyone , I Am Sophie Martin . I Am a content Writer . and I would like Show You Suggest You Some Updates Regarding Hp Printer Support Phone Number . If you want to resolve your Any Problem Regarding Hp Printer support Number So You Can Visit On My website Hp Printer Support Number Tech
ReplyDelete"
I have been looking forward to having someone I can rely on, in terms of information. The information I have gotten from this blog, I must say that I'm in the right place. unilorin cut off mark for computer science
ReplyDeleteThis is a really good tip particularly to those fresh to the blogosphere.
ReplyDeleteShort but very accurate information… Appreciate your sharing this one.
A must read post!
경마
magosucowep
You’re so interesting! I don’t believe I’ve truly read something like this before. So great to find someone with genuine thoughts on this issue. Really.. many thanks for starting this up. This website is something that’s needed on the internet, someone with some originality!
ReplyDeleteCBSE Schools In Ahmedabad
CBSE Schools In Surat
CBSE Schools In Rajkot
CBSE Schools In Visakhapatnam
CBSE Schools In Kangra
CBSE Schools In Shimla
CBSE Schools In Jammu
CBSE Schools In Solan
CBSE Schools In Mangalore
CBSE Schools In Mysore
amitabh bachchan zodiac sign Planetary Combinations. Rahu is traversing the tenth house to his ascendant sign in his Surya Kundali, while Ketu and Saturn are transiting.
ReplyDeleteThe above information you shared give me a new look at this topic. I am working as a full-time academic writer in myassignmenthelp, which provides assignment writing services to college students studying in Australia.
ReplyDeleteHard exams, assignments and homework are a now things of the past with Drug Use And Abuse 7th Edition Test Bank. Order and download now!
ReplyDeleteThe best source for learning today are online resources. Students can find the best Test Banks and Solution Manuals Online including Test Bank For Hospitality Facilities Management And Design 4th Edition at TestBanks21.
ReplyDeleteIf you are in a tight fix with an exam or homework, TestBanks21 could be the solution you are looking for. Browse through our vast collection of test banks and solution manuals like Marketing Of High Technology Products And Innovations 3rd Edition Solution Manual Browe now for better grades!
ReplyDeleteExcellent blog, very clear and informative. Thank you for an outstanding content. Check LMU post utme past question online
ReplyDeleteCropaa is the best company in India for providing Mobile Accessories. It also provides the mobile cover for many companies like Oppo, Vivo, Samsung, Realme, etc. it provides all products all over India. If any customer wants the best Online Shop For Mobile Accessories then he can visit the Cropaa website and choose the mobile accessories according to the requirements.
ReplyDeleteAt GlobalAssignmentexpert.com, we never compromised with the quality.Our writers prepare a top-notch assignment for you that is free from any sort of plagiarism. We ensure you that you will score perfect grades if you are taking our perdisco assignment help.
ReplyDeleteSolutions manual and Test Banks Online are the easiest tools to increase your college grades instantly. Get a 4.0 on your exams with Test Bank For Give Me Liberty An American History 3rd Edition today.
ReplyDeleteHard exams, assignments and homework are a now things of the past with Solution Manual For Basic College Mathematics 5th Edition. Order and download now!
ReplyDeleteThe content on this Iperius Backup Crack POST is excellent. You can download crack software for Mac and Windows from CRACKGRID Site.
ReplyDeleteThanks for sharing this usefull topic. i hope it is improtant
ReplyDeleteiobit uninstaller pro crack post for everybody. thanks and have a good day
wincatalog crack beautiful site
ReplyDeleteYou have done a great job on this article. It’s very readable and highly intelligent. You have even managed to make it understandable and easy to read Directory Lister Pro Full Keygen
ReplyDeletevery nice.keep it up good work.Brorsoft Video Converter Crack
ReplyDeleteThis post is a pool of information. The readers are quite impressed after reading this impressive post.
ReplyDeleteGlassWire Elite
Corel PhotoMirage License Key
ReplyDeleteGreat Article. Looking great work dear, I really appreciated to you on this quality work. I would like say thanks for this post. Here are some useful links for students all across the globe. These tips may help me in the future. A high-standard post with all imperative information.
Thank you for writing such a great informative article for today's modern readers. Two thumbs up for great content and interesting views.
ReplyDeleteivt bluesoleil crack
ReplyDeletewhat a informative and knowlegeable websites.Luminar 2018 Crack
very nice.keep it up good work.Pdfescape Crack
ReplyDeleteThis is a wonderful website and I enjoy your writing style.
ReplyDeleteI appreciate you sharing this informative post with us.
easeus data recovery torrentz2
idm crackYet forward bends can also be a challenge to many people, especially those with tight hamstrings.
ReplyDeleteRaise your right arm overhead and bend your upper body to the left in a reaching motion. Keep your upper body facing straight ahead—don't twist it to the side as you bend. ableton live suite
ReplyDeleteBellsouth.net Email Login
ReplyDeleteBellsouth Email Login
what a informative and knowlegeable websites.Nikon Camera Control Pro (2.34.2) Serial Key
ReplyDeletewhat a informative and knowlegeable websites.DU Meter full crack,
ReplyDeleteThis article is just what i needed in time. Thank you for having the time to share this with me. Pastquestionpdf is a great way for students to get informed on;
ReplyDeleteBowen University Post UTME form
Bingham University Post UTME Form
Benson Idahosa University Post UTME Form
Bells University Post UTME Form
Baze University Post UTME Form
ReplyDeleteNice post, Keep it up. Thanks for sharing.
Spotify Premium Crack
MiniTool Power Data Recovery Crack
Yousician Crack
adana
ReplyDeleteadıyaman
afyon
ağrı
aksaray
amasya
ankara
ardahan
artvin
This is one of the best website I have seen in a long time thank you so much, thank you for let me share this website to all my friends. 카지노사이트
ReplyDelete
ReplyDeleteNice Blog Post!! Three linking words and, but, or.And if you have questions where should I can use this for that go to my post and see the example.
Very nice post. keep going by posting such great contents. Very interesting and meaningfull content
ReplyDeletecowkart
cowkart
cowkart
Hello there, I just discovered your blog on Google, and I like it.
ReplyDeleteis quite useful. I'll keep an eye out for brussels sprouts.
If you keep doing this in the future, I will be grateful. A large number of people will profit.
based on your writing Cheers!
nuance omnipage ultimate crack
adobe flash player crack
snapshot pro crack
serif affinity designer crack
ReplyDeleteThanks for Sharing such an amazing article. Keep working... Your Site is very nice, and it's very helping us.. this post is unique and interesting, thank you for sharing this awesome information crackpro.co
Your presentation is very easy to follow, yet I don't like it.
ReplyDeleteI'm sure I'll never be able to solve this issue.
It appears to me to be extremely complicated and wide-ranging.
I can't wait to read your next post. I make an effort at this.
agree!
ultraiso crack
tally erp 9 crack
windows 8 activator crack
glary utilities crack
I'm glad you like it as much as I do.
ReplyDeleteA sketch, a theme for your author, here.
elegant shopping
Lack of interest in what you are saying.
You will return earlier and will return much longer if you help us with the expansion.
gstarcad pro crack
pluraleyes crack
audials one crack
sparkol videoscribe full crack
I have enjoyed it very much. Thanks for your website.
ReplyDeleteI think the website is GREAT!
It is easy to follow along and definitely convienent
driver easy pro crack
4k stogram crack
driver genius crack
iobit smart defrag crack
Your writing and presentation skills as well as your overall style have really impressed me.
ReplyDeleteblog A purchased theme or one that you've modified is either the case.
You? Whatever the situation may be, the most important thing is to listen to new music with high-quality lyrics.
Today, I seldom come across a blog as nice as this one.
reaper crack
xsplit gamecaster studio crack
youtube movie maker crack
diskdigger crack
flexisign pro crack latest version
virtual dj crack
avg internet security crack key
avs video editor
I'm really impressed with your writing skills, as smart as the structure of your
ReplyDeleteLatest Software Free Download
weblog. Is this a paid topic
Diskdigger crack
do you change it yourself? However, stopping by with great quality writing, it's hard to see any good blog today.
Program4pc audio convertercrack
Diskdigger-crack
Reimage pc reapir crack
adobe dreamweaver pro crack
I'm truly enjoying reading this post of yours.
ReplyDeleteGreat lighting and work!
Keep up the good work, and thanks for sharing.
reimage pc repair crack
ashampoo antivirus crack
visual studio product key crack
aurora hdr crack
DangalGames is the best place for you to be entertained and to make some earnings with online real money earning games in India. Are you looking for a fun way to kill time and earn money through games? You can easily earn money playing games on DangalGames! There is a chance that you can play free online games to earn money in India.
ReplyDeleteearn money by playing games
play game and earn money online
The real online money earning games help people to play with live competitors and meet the new people who also love the same game. Having these people who share the same interest in gaming would give you new friends and some people who would play the money earning games with you. People from all around the world come to play these real cash games so you can meet all those people on the online platform.
ReplyDeleteplay games earn money
real cash earning app
FantasyDangal has become one of the most popular new fantasy app. In a very short time, the FantasyDangal app has gained a lot of popularity. This new fantasy cricket app is available in nine languages and offers a range of bonuses and offers to users who want to play fantasy cricket, win cash, and stay in the game. The app also offers different modes such as Safe Play and Regular play that make it easy for users.
ReplyDeleteVery Good blog. A good Effort from the Author Side. The Best SMM Pak Panel provides the powerful promotion of social media accounts to enable you to Beat your competitors. We would be pleased to grow your business with our best SMM panel services. Visit us for. Total SMM Panel
ReplyDeleteThis absolutely amazing, you got true and fresh information posted here, really found it useful, thanks for posting and the effort! Please keep sharing more of such blogs. adamawa state coe cut off mark for social studies/yoruba
ReplyDeletenice post
ReplyDeleteABBYY FineReader Crack
Revo Uninstaller Pro Crack
Movavi Screen Recorder Crack
This is a great idea, especially for those who have just started blogging.
ReplyDeleteInformative despite its simplicity.
It's a pleasure to read.
If you have not read this article, please.
auslogics boostspeed premium with crack
qtranslate crack
screenpresso pro crack
toon boom storyboard pro crack
pro evolution soccer crack
I am very impressed with your post because this post is very beneficial for me and provide a new knowledge to me. this blog has detailed information, its much more to learn from your blog post.I would like to thank you for the effort you put into writing this page.
ReplyDeleteI also hope that you will be able to check the same high-quality content later.Good work with the hard work you have done I appreciate your work thanks for sharing it. It Is very Wounder Full Post.This article is very helpful, I wondered about this amazing article.. This is very informative.
“you are doing a great job, and give us up to dated information”.
aiseesoft video converter ultimate crack/
es computing editplus crack/
sparkbooth dslr/
advanced systemcare download/
graphpad prism free/
you for the information you provide, it helped me a lot. Thanks for Awesome tips Keep it up
ReplyDeleteKeep up the good work. And Thanks For Sharing
Nero Burning ROM Crack
VMware Fusion Pro Crack
Arclab Watermark Studio Crack
Goversoft Privazer Crack
GlarySoft Malware Hunter Pro Crack
AnyMP4 Video Converter Ultimate Crack
Adobe Audition CC Crack
Ramadan gifts refers to a child’s first fast when they are she is old enough. It is a significant and quite a popular ritual. For the child, it is a thrilling experience. Although the concept of going without food can be difficult for children to grasp, they are ecstatic to embark on their first fast. Hence many parents and siblings choose to enhance their loved one’s Roza Kushai celebration with premium Roza Kushai gifts and top-quality Ramazan balloons
ReplyDeleteroza kushai gifts
ReplyDeleteIt doesn't take a ton of delving to uncover the perfections in this write-up. I'm sure this blog will be lot of usefulness to all mothers. Thanks for sharing. Also, check sterling bank recruitment process
ReplyDeleteI guess I am the only one who came here to share my very own experience. Guess what
ReplyDeleteI am using my laptop for almost the past 2 years, but I had no idea of solving some basic issues.
I do not know how to Crackadvise Free Download But thankfully, I recently visited a website named Crackadvise.Crack
Magoshare Data Recovery Crack
CyberGhost VPN Crack
Mastercam Crack
CCleaner Professional Key Crack
MediaHuman YouTube Downloader Crack
DecSoft App Builder Crack
Boom 3D Crack
Thank you so much for sharing this great blog.Very inspiring and helpful too. You can also go through my latest article Spacebar speed test this speed test tool, you can take your skills of striking the space bar key this might be a great help to you.
ReplyDeleteReally inspired from your writing skill. Nice efforts.
ReplyDeleteDataNumen RAR Repair Crack
Very nice article. I definitely love this website.Great Article. Thanks For sharing.
ReplyDeleteAutodesk Revit Crack
Drug Synthesis International: Ostat Series >>>>> Download Now
ReplyDelete>>>>> Download Full
Drug Synthesis International: Ostat Series >>>>> Download LINK
>>>>> Download Now
Drug Synthesis International: Ostat Series >>>>> Download Full
>>>>> Download LINK 4c
Thank you for sharing this fantastic blog. It is loaded with important information. Keep it up!
ReplyDeletehttps://keygenhere.com/dgflick-album-xpress-pro-12-0-crack/
After reviewing some of the ads on your site,
ReplyDeleteWe really appreciate the way you write your blog.
We've added it to our Bookmarks website list and we'll check back soon.
Future. Also check out my website and let us know what you think.
You did a good job and worked hard. I appreciate your work, thanks for sharing.
PTE AV Studio Pro Crack
EZ CD Audio Converter Crack
Little Alter Boy Creek
Abelssoft CheckDrive Pro Crack
MixPad Masters Edition Crack
Movavi Video Suite Crack
Enscape3D Crack
Microsoft Excel Crack
I am a professional web blogger so visit my website link is given below!To get more information
ReplyDeletecrackroot.net/
PhpStorm Crack/
Dr.Fone Crack/
Substance Painter Crack/
DevExpress Universal Crack/
iZotope Ozone Crack/
Avira Optimization Suite Crack/
InstallShield crack/
Tally ERP 9 Crack/
Computer Science Solutions based on your textbook is the smartest way to handle your homework, projects, assignments and exam preparation. Checkout the ScholarOn collection to be on the top of your class.
ReplyDeleteWow.. Thank you for offering such an informative article.
ReplyDeletehttps://easyserialkeys.com/disk-drill-pro-crack-download/
Like many airlines, United airlines baggage policy is complex. The amount of free luggage you receive and how much you pay for baggage are determined by the vacation you are taking. On rare occasions, you can get a complimentary bag. One checked bag can cost $60 or more on occasion. Please follow the link to learn everything you need to know.
ReplyDeleteen son çıkan perde modelleri
ReplyDeleteMobil Onay
turkcell mobil ödeme bozdurma
nft nasıl alinir
ankara evden eve nakliyat
Trafik Sigortası
dedektor
website kurma
aşk kitapları
Magellan roadmate Updates
ReplyDeleteSome popular Gps devices like- Magellan devices like magellan maestro 3200,magellan roadmate 1440,magellan maestro 4040, magellan roadmate 5235t-lm,magellan roadmate 1700,magellan roadmate 1470, and other old and newer models. We’ll help you to get easy maps, software / Firmware updates.
Are you planning a trip or going out and wondering if you could have the latest maps to make your trip or your work. Well, you’re a step away from the latest map updates. Latest map updates make sure that you always reach your destination on time with full-proof accuracy.
The looks really great. Most of these smaller details are usually created employing a wide range of heritage knowledge.
ReplyDeleteactivatorproductkey.com
GridinSoft Anti Malware Crack
Cubase Pro Crack
IntelliJ IDEA Ultimate Crack
Sketch Crack
Visual Studio Crack
I guess I am the only one who came here to share my very own experience. Guess what!? I am using my laptop for almost the past 2 years, but I had no idea of solving some basic issues. I do not know how to Crack Softwares Free Download But thankfully, I recently visited a website named xxlcrack.net/
ReplyDeleteTipard Video Converter Ultimate Crack
Winsoft PDFium Component Suite Crack
Nice & Impressive Blog!https://trustedcrack.com/ez-cd-audio-converter-crack/
ReplyDeleteThanks for blog! Want to submit your assignment before deadline but don't know how to write? then, you should take Nike swot analysis assignment help from BookMyEssay.
ReplyDeleteThis is one of the easiest Wifi password hacking application. This Application is very easy to use and download and easily available Online Wifi Hacker
ReplyDeleteHitman Pro Crack can be an antivirus by Surfright. It is not an old-fashioned antivirus software package but an additional opinion scanner. Hitman Pro Serial Number
ReplyDeleteThanks for your page! Your share information it helped me alot
ReplyDeletecrankshaft polishing
crankshaft repair
ReplyDeleteThanks for your page! Your share information it helped me alot
crankshaft grinding services
portable crankshaft grinder
Thanks for your page! Your share information it helped me alot
ReplyDeletecylinder liners exporter
white metal babbitt bearing
Thanks for your page! Your share information it helped me alot
ReplyDeletecrankshaft grinder
portable crankshaft grinding machine
NEET is the best option to make your dream come true. On the other hand, All India Institutes of Medical Sciences (AIIMS) and Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) are allowed to conduct separate public placement tests for admission to MBBS courses through their assessment. This evaluation depends on the class 11 and 12 schedule. Without more, they can crack the exam if they keep all the concepts in mind and take enough mock tests. NEET Coaching Center in Delhi is an exclusive clinical selection test in the country for admission to clinical and dental college classes. For all medical universities, the government should admit students based on NEET rank and accuracy. For this we have to take the NEET exam first. While self-study is an amazing preparation option, the added expert help of coaching centers can help you benchmark your performance against others and guide you in a better time. However, before we go into the list of institutions, how do we find the answers to the questions that must be considered before visiting or choosing an institution? Everyone is looking for the best coaching institutes. No one wants to join a lame coaching where the faculty and facilities are not good. So, Digitalcoachedu.com brings you a list of the best NEET coaching institutes in Delhi that you should join if you really want to crack the NEET exam. This is an amazing exam which is conducted in every NEET coaching center in Delhi.
ReplyDeleteSee more- Top 10 Best NEET Coaching In Delhi
Your writing style is very distinctive compared to those of other authors I've read. Thank you for posting when you get the chance.
ReplyDeletewolfram mathematica crack
apowermirror crack
ashampoo soundstage pro crack
daemon tools pro crack
Ireland Assignment Helper is the premier provider of cheap essay writing services in Ireland . Our team of professional writers takes pride in creating essays that help students succeed and understand the material they are studying. We offer top-notch quality, great customer service, and affordable prices to ensure that you get the most value for your money!
ReplyDeleteNice Article very glad to read your Article
ReplyDeletepython training in hyderabad
Good blog
ReplyDeleteLooking for reliable translation services in UK? Look no further! My Translation Services is your go-to destination for accurate and professional language translations. With a team of experienced linguists, we offer a wide range of services, including document translation, website localization, and interpreting. Whether you need business translations or personal documents translated, our experts ensure quality and timely delivery. Visit our website, My Translation Services, to explore our comprehensive language solutions tailored to meet your specific needs.
Your articles on black Satta King were really educational for me. Knowing the game's origins is crucial. It is intriguing to see the government regulate gambling in some state after decades of debate.Your recommendation to go slow in the beginning is a sensible and reasonable way to avoid major setbacks. People should use caution and consider the risks associated with these games.I appreciate youeffort and the time to explain the ins and outs of Satta King. You blog are complete package if info who want to start its journey.
ReplyDeletePanobinostat is a cinnamic hydroxamic acid analogue with potential antineoplastic activity. Panobinostat selectively inhibits histone deacetylase (HDAC), inducing hyperacetylation of core histone proteins, which may result in modulation of cell cycle protein expression, cell cycle arrest in the G2/M phase and apoptosis. Most students are drawn to these types of articles and information, but they are unable to prepare for their exams, If you have been struggling with your exams and want assistance, students can hire test takers online and get higher grades on their examinations by providing them with the best available resources, including quality academic services.
ReplyDeletePanobinostat is a histone deacetylase (HDAC) inhibitor which was filed for approval in the U.S. in 2010 for the oral treatment of relapsed/refractory classical Hodgkin’s lymphoma in adult patients. The company is conducting phase II/III clinical trials for the oral treatment of multiple myeloma, chronic myeloid leukemia and myelodysplasia. Most students are drawn to these types of articles and information, but they are unable to prepare for their exams, If you have been struggling with your exams and want assistance, students can take online class help and get higher grades on their examinations by providing them with the best available resources, including quality academic services.
ReplyDeletePanobinostat is a cinnamic hydroxamic acid analogue with potential antineoplastic activity. Panobinostat selectively inhibits histone deacetylase (HDAC), inducing hyperacetylation of core histone proteins, which may result in modulation of cell cycle protein expression, cell cycle arrest in the G2/M phase. Most students are drawn to these types of articles and information, but they are unable to prepare for their exams, If you have been struggling with your e-commerce project and want assistance, students can visit One Digital Stop and get the best performance on their website by providing them with the most excellent available resources, including quality web design services.
ReplyDeleteUnveil the healing potential within you through Healing Buddha, where the ancient wisdom of energy healing merges with modern expertise.
ReplyDeletepranic healing
I see your blog its very interesting for me and relevant to my topic.
ReplyDeletePlease Visit my Blog Page MATLAB R2023A Crack
Very Interesting Blog. I like it very much. For relevant blog please visit MATLAB R2023A Crack
ReplyDeleteNice blog and Good information, Keep posting more.
ReplyDeleteAWS DevOps institute in KPHB
Nice Info,Please visit : Sbobet88
ReplyDeleteResolve Exodus Log Errors with Ease! Our troubleshooting guide provides simple solutions to tackle any log errors you encounter in Exodus. Stay in control of your crypto experience – explore our guide today and bid farewell to Exodus log errors for a seamless wallet management journey.
ReplyDeleteInsomnia or Sleepiness: Lexapro can affect sleep patterns, leading to either difficulty falling asleep or excessive drowsiness. Establishing a consistent sleep schedule, practicing good sleep hygiene, and discussing potential adjustments to your medication regimen with your healthcare provider can help address these issues.
ReplyDeletelexapro-uses-dosage-side-effects-warnings
Uncover the Secrets of Bitcoin Mining! Learn How to Mine Bitcoin with our comprehensive guide. Whether you're a novice or seasoned miner, our instructions ensure a smooth mining process. Take control of your crypto journey – explore our guide today and delve into the world of Bitcoin mining.
ReplyDeleteWhich Crypto Faucets Require The Least Time Commitment
ReplyDeleteMobile crypto faucets with high earning potential
Earn rewards on different faucets
Earn rewards on different faucets
Unlock Seamless Blockchain Connection Solutions! Resolve Cannot Connect to the Blockchain issues with our expert guide. Explore troubleshooting tips and ensure uninterrupted access to the blockchain. Stay connected and streamline your crypto experience. Discover solutions today!
ReplyDelete