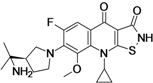
ACH-702
7-[3(R)-(2-Aminopropan-2-yl)pyrrolidin-1-yl]-9-cyclopropyl-6-fluoro-8-methoxy-2,3,4,9-tetrahydroisothiazolo[5,4-b]quinoline-3,4-dione
(7^-7-[3-(1-AMrNO-I-METHYLETHYL)PYRROLiDiN-I-YL]-P-CYCLOPROPYL-6-FLUORO-8-METHOXY-PH-ISOTHIAZOLO[5,4-B]QUINOLINE-3,4-DIONE
(R)- 7-[3-(l-amino-l-methyl-ethyl)-pyrrolidin-l-yl]-9-cyclopropyl-6-fluoro-8-methoxy- 9H-isothiazolo[5, 4-b] -quinoline-3, 4-dione
922491-46-1 free base
922491-09-6 (hydrochloride)468.973, C21 H25 F N4 O3 S . Cl H
ACH-0139586
ACH-702
ACH-702
Achillion Pharmaceuticals (USA)

pre clinical
Achillion Pharmaceuticals is working on the discovery of compounds in a new subclass of quinolones, the isothiazoloquinolones. The most advanced compound is ACH-702, which is at the pre-clinical stage of development [1-3].
 ACH 702
ACH 702
The utility of isothiazoloquinolines as pharmaceutical agents has been discussed in the literature. For example, Pinol, et al discussed the use of isothiazoloquinolines as medical bactericides in US Patent 5,087,621, including
The Proctor & Gamble Company discussed antimicrobial quinolones including the following compound:
in published application no. US 2003008894.
The use of isothiazoloquinoline compounds as TNF production inhibitors has also been discussed, for example by Sankyo Co., Ltd. in JPl 010149, which includes the following compound
Bayer Aktiengesellschaft has discussed bicycle[3.3.0]oct-7-yl containing compounds useful for treating H. pylori infections in WO 98/26768, including isothiazoloquinolines, having the general structure shown below in which Y may be sulfur joined to the carboxamide group to form a 5-membered ring
Otsuka Pharmaceutical Co., Ltd. has discussed the use of isothiazoloquinolines as antibacterial agents in JP 01193275, including the following carbamate-containing compound
Abbott Laboratories has discussed the use of isothiazoloquinolines as antineoplastic agents in US Patent No. 5,071,848 and has discussed the use of tricyclic quinolones as antibacterial agents in US 4,767,762. The Abbott compounds have hydrogen, halogen, or lower alkyl as substituents at the 6- and 8- positions of the isothiazoloquinoline core.
..................
Synthesis
WO2008021491A2
http://www.google.com/patents/WO2008021491A2?cl=en
EXAMPLE 1. SYNTHESIS OF (7^-7-[3-(1-AMrNO-I-METHYLETHYL)PYRROLiDiN-I-YL]-P-
CYCLOPROPYL-6-FLUORO-8-METHOXY-PH-ISOTHIAZOLO[5,4-B]QUINOLINE-3,4-DIONE (5). Step 1. Ethyl l-cyclopropyl-6, 7-difluoro-2-methanesulfonyl-8-methoxy-4-oxo-l,4-dihydro- quinoline-3-carboxylate (6)
Oxonβ®
MeOHZH2O
1
6
Water (180 mL), followed by Oxone® (Dupont Specialty Chemicals) (170 g, 277 mmol), is added to a suspension of 1 in MeOH (510 mL). The reaction mixture is heated with stirring at 55-60 0C for 3 h. The reaction mixture is cooled to room temperature, diluted with water (40 mL), and stirred at 5 0C (ice bath) for 30 min. The resulting crystals are collected by filtration, washed with water (2 x 100 mL), and dried to afford 6 (13.8 g). This material was used in the next step without further purification, mp 177-178 0C. 1H NMR (DMF-^7): J0.62 (m, IH), 1.11 (m, 2H), 1.29 (m, IH), 1.32 (t, JH-H = 7.0 Hz, 3H), 3.76 (s, 3H), 4.18 (m, IH), 4.21 (d, JH-F = 2.0 Hz, 3H), 4.33 (q, JH-H = 7.0 Hz, 2H), 7.64 (dd, JH-F = 10.0 Hz, 8.5 Hz, IH). Step 2 (R)- 7-[3-(l-amino-l-methyl-ethyl)-pyrrolidin-yl]-l-cyclopropyl-6-fluoro-2- methanesulfonyl-8-methoxy-4-oxo-l , 4-dihydro-quinoline-3-carboxylic acid ethyl ester (7)
10 6 7
A mixture containing compound (6) (3.88 g, 9.67 mmol), compound 10 (1.64 g, 12.8 mmol), anhydrous DIEA (5.05 g, 39.1 mmol, dried over 4A sieves), and anhydrous DMF (40 mL) is heated at 70 0C under an atmosphere of argon gas. After heating for 4.5 h (LC-MS analysis shows ~7% compound (6) remained), the reaction mixture is cooled to room temperature, diluted with EtOAc (200 mL), and washed with water (100 mL). The aqueous layer is extracted with EtOAc (100 mL), and the combined organic layers are washed with a saturated aqueous solution of sodium bicarbonate (100 mL). The organic layer is diluted with water (100 mL) and treated with an aqueous solution of HCl (4 N) until the aqueous layer is acidic (pH 2—3 after shaking the mixture vigorously). The organic layer is separated, and this process is repeated. The combined aqueous layers are diluted with EtOAc (100 mL) and treated with an aqueous solution of sodium hydroxide (6 N) until the aqueous layer is basic (pH ~8 after shaking the mixture vigorously). The aqueous layer is separated, and this process is repeated. The combined organic layers are dried over magnesium sulfate, filtered, and concentrated under reduced pressure giving an orange solid (3.27 g of an~80:20 mixture of compound (7) and impurity B). This solid is recrystallized from hot EtOAc (~60 mL) furnishing 2.18 g (44% yield) of pure compound 7 as a bright yellow solid. LC-MS mlz calcd for C24H32FN3O6S 509 ([M+]); found 510 ([M + H]+).
This reaction should not be allowed to proceed for more than a few hours (not overnight) as prolonged reaction time can lead to the formation of more side products. The product should be —95% pure (based on HPLC), with only a trace amount of impurity B. Step 3. (R)-7-[3-(l-amino-l-methyl-ethyl)-pyrrolidin-yl]-l-cyclopropyl-6-fluoro-2-mercapto-8- methoxy-4-oxo-l,4-dihydro-quinoline-3-carboxylic acid ethyl ester (8)
7 8
Compound 7 (1.04 g, 2.04 mmol) is partially dissolved in DME (40 mL) under an atmosphere of argon. Sodium hydrosulfide hydrate (Aldrich, 72.6% by titration, 465 mg, 6.02 mmol) in water (3.0 mL) is added to this solution. The resulting mixture is sparged slowly with argon for 30 min.
The progress of the reaction is monitored by HPLC-MS, and judged to be complete (<2% of 7 remains) after 11.5 h. Excess sodium hydrosulfide is quenched upon addition of aq HCl (4.5 mL, 4 N).
The resulting orange solution (pH ~2) is sparged with argon (30 min) to remove the generated hydrogen sulfide. Step. 4 (R)- 7-[3-(l-amino-l-methyl-ethyl)-pyrrolidin-l-yl]-9-cyclopropyl-6-fluoro-8-methoxy- 9H-isothiazolo[5, 4-b] -quinoline-3, 4-dione (5)
5= ACH 702
A solution of potassium carbonate (4.26 g, 30.8 mmol) in water (25 mL) is next added to this solution to give a clear yellow solution (pH 9-10). The clear yellow solution is then sparged with argon for ~5 min. Finally, hydroxylamine-0-sulfonic acid (0.93 g, 8.2 mmol) is added portionwise as a solid, with immediate evolution of gas and formation of the product as a yellow precipitate. After stirring for 16 h, the reaction mixture (pH 10.2) is acidified with aq HCl to pH 8.3 (the approximate isoelectric point of 5) causing additional product to precipitate from solution. The reaction mixture is concentrated under reduced pressure (final volume -40 mL). The yellow precipitate is collected by centrifugation, washed with water (3 x 40 mL, with sonication), and lyophilized to give 0.80 g of 5.
..........................
WO2007014308A1
http://www.google.com/patents/WO2007014308A1?cl=en
EXAMPLE 5. SYNTHESIS OF I-METHYL-I-PYRROLIDIN-3-YL ETHYLAMINE (5)
1 -Methyl- l-pyrrolidin-3-yl-ethylamine is prepared in accordance with the synthetic scheme below.
N O
5 P
Step 1. Synthesis of (S)-l-benzylpyrrolidin-3-yl methanesulfonate (N).
Methanesulfonyl chloride (15 mL, 0.19 mol) is added to a cooled (0 0C) solution of toluene (300 mL) containing (5)-l-benzylpyrrolidin-3-ol (24.5 g, 0.14 mol) and triethylamine (80 mL, 0.57 mol). The resulting mixture is stirred at 0 °C for 15 min, and allowed to warm to room temperature with stirring for 2h. The mixture is quenched with a 5% aqueous solution of sodium bicarbonate (250 mL). The organic layer is washed with a 5% aqueous solution of sodium bicarbonate (2 x 250 mL), washed with water (I x 250 mL), dried over magnesium sulfate, and concentrated under reduced pressure to give N (35.1 g, 99 %) as an orange oil. 1H NMR (300 MHz, CDCl3): £2.07 (m, IH), 2.30 (m, IH), 2.49 (m, IH), 2.75-2.90 (m, 3H), 2.98 (s, 3H), 3.61 (d, J= 13.0 Hz, IH), 3.68 (d, J= 13.0 Hz, IH), 5.18 (m, IH), 7.15-7.30 (m, 5H). LCMS mlz calcd for C12H17NO3S 255 ([M+]); found 256 ([M + H]+, 100%), 160 (40%). Steps 2 and 3. Syntheses of(R)-l-benzylpyrrolidine-3~carbonitrile (O) and 2-((R)-I- benzylpyrrolidin-3-yl)propan-2-amine (P).
The syntheses of O and P are described previously by Fedij et al. (Fedij, V.; Lenoir, E. A., Ill; Suto, M. J.; Zeller, J. R.; Wemple, J. Tetrahedron: Asymmetry 1994, J, 1131- 1134). Step 4. Synthesis ofl-((R)-Methyl~l-pyrrolidin-3-yl)-ethylamine (5).
A mixture containing P (7.4 g), 20% palladium hydroxide on carbon (7.5 g), and ethanol (75 niL) is stirred under an atmosphere of hydrogen gas (50 psi) at 45 °C for 24 h. The mixture is filtered and the filtrate is concentrated under reduced pressure to give 5 (4.1 g, 95 %) as a yellow oil. This material is stored under an atmosphere of argon gas. 1H NMR (300 MHz, CDCl3): J1.09 (s, 6H), 1.51 (m, IH), 1.64 (br s, 3H), 1.81 (m, IH), 2.06 (apparent pentet, J= 8.5 Hz, IH), 2.69 (dd, J= 11.0 Hz, J= 8.5 Hz, IH), 2.94 (m, 2H), 3.00 (dd, J= 11.0 Hz, J= 8.5 Hz, IH). LCMS mlz calcd for C7H16N2 128 ([M+]); found 129 ([M + H]+, 60%), 112 (100%).
EXAMPLE 6. GENERAL METHOD FOR THE FINAL AMINE-COUPLING STEP: SYNTHESIS OF 7-((R)-3-
(2-AMINOPROPAN-2-YL)PYRROLIDIN- 1 -YL)-9-CYCLOPROPYL-6-FLUORO-8- METHOXYISOTHIAZOLO[5,4-B]QUINOLINE-3 ,4(2H,9H)-DIONE HYDROCHLORIDE
[0261 ] 7-((R)-3-(2-Aminopropan-2-yl)pyrrolidin- 1 -yl)-9-cyclopropyl-6-fluoro-8- methoxyisothiazolo[5,4-b]quinoline-3,4(2H,9H)-dione hydrochloride is prepared in accordance with the synthetic scheme below.
Synthesis ofJ-ffRJS-^-aminopropan^-ylJpyrrolidin-l-ylJ-P-cyclopropyl-o-fluoro-S- methoxyisothiazolofS, 4-bJguinoline-3,4(2H, 9H)-dione hydrochloride (6).
Under an atmosphere of argon, a reaction vessel is charged with 5 (206.0 mg, 1.6 mmol), 3 (328.6 mg, 1.0 mmol), dimethyl sulfoxide (4.5 mL), and ΛζN-diisopropylethylamine (750 μL, 4.3 mmol). The resulting mixture is irradiated with microwaves (CEM Discover) at 125 0C for 1 h (conventional heating may also be used — 115 °C in an oil bath for 14 h), allowed to cool, and evaporated to dryness under reduced pressure (-70 °C/2-3 mm Hg). The oily residue is triturated with ethyl acetate (15 mL) and the resulting powder is collected by centrifugation. This solid is purified using preparative HPLC to give the desired product. Preparative HPLC is performed using a YMC Pack Pro C18 150 x 30.0 mm 5//m column coupled to a YMC Pack Pro 50 x 20 mm 5/an column with an isocratic elution of 0.37 min at 95:5 H2OiCHsCN containing 0.1% TFA followed by a 15.94 min linear gradient elution from 95:5 to 25:75, followed by a 0.69 min linear gradient from 25:75 to 5:95 at a flow rate of 30.0 mL/min with UV detection at 254. The crude material is loaded as a solution containing acetic acid (~2 mL), methanol (~1 mL), and water (~1 mL). The purified product is isolated as the TFA salt and is converted to the corresponding hydrochloride salt by addition of a solution of hydrogen chloride (~1.25 M in methanol) followed by evaporation; this process is repeated twice to give a yellow solid. Purity by HPLC: >99%; tR = 10.08 min. 1H NMR (300 MHz, TFA-d): δ 1.28 (m, 2H), 1.53 (m, 2H), 1.66 (s, 6H), 2.43 (m, IH), 2.57 (m, IH), 3.35 (m, IH), 3.97 (s, 3H), 4.01-4.38 (m, 5H), 8.17 (d, J= 12.0 Hz, IH, aromatic). 19F(1H) (282 MHz, TFA-J): δ-\ 18.0 (s). 13C(1H) (75 MHz, TFA-d): £13.5, 13.9, 25.0, 25.1, 29.1, 39.7, 49.6, 59.4 (br, W1/2 « 14 Hz), 59.8 (br, W1/2 « 14 Hz), 60.0, 66.8, 106.0, 112.1 (dJc_F = 23.0 Hz), 137.5 (br m, W1/2 « 24 Hz), 138.4, 144.8 (br, W1/2 » 10 Hz), 155.3 (dJc_F = 255.0 Hz), 169.8, 170.1, 171.5 (br, W1/2 « 9 Hz). LCMS mlz calcd for C21H25FN4O3S 432 ([M+]); found 433 ([M + H]+). Anal. Calcd for C21H25FN4O3S-l.5HCM.5H2O: C, 49.05; H, 5.78; N, 10.90; Cl, 10.34. Found: C, 49.30; H, 5.60; N, 10.83; Cl, 10.00.
EXAMPLE 3. SYNTHESIS OF 9-CYCLθPRθPYL-6,7-DiFLUθRθ-8-METHθχγ-9H-isoτHiAzθLθ[5,4- 5]QUlNOLlNE-3,4-DIONE (Compound 3).
9-Cyclopropyl-6,7-difluoro-8-methoxy-9H-isothiazolo[5,4-&]quinoline-3,4-dione (3) is prepared in accordance with the synthetic scheme below.
Step 1. Synthesis of 2,4, 5-trifluoro-3-methoxybenzoyl chloride (A)
A mixture of 2,4,5-trifluoro-3-methoxybenzoic acid (154 mg, 0.75 mmol) and thionyl chloride (8 mL) is refluxed for 4 h. Excess thionyl chloride is removed in vacuo, and the remaining residue is used directly in the next synthetic step. Step 2. Synthesis of (Z)-ethyl 3-hydroxy-3-(2,4,5-trifluoro-3-methoxyphenyl)aaγlate (B).
Compound B is prepared using the general method of Wierenga and Skulnick (Wierenga, W.; Skulnick, H. I. J. Org. Chein. (1979) 44: 310-311). H-Butyllithium (1.6 M in hexanes) is added to a cooled (-78 °C) solution of tetrahydrofliran (10 mL) containing ethyl hydrogen malonate (180 juL, 1.50 mmol) and 2,2'-bipyridyl (~1 mg as indicator). The temperature of the reaction mixture is allowed to rise to ca. -5 0C during the addition of n- butyllithium. Sufficient n-butyllithium (2.8 mL, 4.48 mmol) is added until a pink color persists at -5 0C for 5-10 min. A solution of 2,4,5-trifluoro-3-methoxybenzoyl chloride (0.75 mmol, vide supra) in tetrahydrofuran (~3 mL) is added in one portion to the reaction mixture that had been recooled to -78 0C. The resulting mixture is allowed to warm to room temperature, diluted with ethyl acetate (50 mL), and quenched with a 1 M aqueous solution of hydrochloric acid. The organic layer is washed with a 5% aqueous solution of sodium bicarbonate (2 x 30 mL), followed by brine (2 x 50 mL), dried over sodium sulfate, and evaporated under reduced pressure to give the crude product. This material is purified by flash column chromatography (eluting with 20% v/v ethyl acetate in hexanes) to give pure B as a white solid. 1H NMR (300 MHz, CDCl3): (enol, predominant tautomer, >90%) δ 1.32 (t, JH-H = 7.0 Hz, 3H, CO2CH2CH3), 4.02 (apparent t, JH-F = 1.0 Hz, 3H, OCH3), 4.25 (q, JH-H = 7.0 Hz, 2H, CO2CH2CH3), 5.79 (s, IH, CH3C(OH)=CH- CO2CH2CH3), 7.39 (ddd, JH_F= 11.0 Hz, 8.5 Hz, 6.5 Hz, IH, aromatic), 12.68 (s, IH, OH). 19F(1H) NMR (282 MHz, CDCl3): <5-146.8 (dd, JF_F = 21.5 Hz, 10.5 Hz, IF), -140.2 (dd, JF_F = 21.5 Hz, 13.5 Hz, IF), -131.3 (dd, JF_F = 13.5 Hz, 10.5 Hz, IF).
Step 3. Synthesis ofζEyethy^-^ZJ-N-cyclopropy^methylthioJcarbonoimidoylJS-hydroxyS- (2, 4, 5-trifluoro-3-methoxyphenyl)acrylate (C)
Sodium hydride (60% in mineral oil, 31 mg, 0.78 mmol) is added portionwise to a cooled (0 °C) solution containing B (200 mg, 0.73 mmol), cyclopropyl isothiocyanate (120 /JL, 1.2 mmol), and dimethylformamide (2 mL). The resulting mixture is allowed to warm to room temperature with stirring overnight (18 h). Methyl iodide (80 juL, 1.2 mmol) is added to the resulting solution and stirred for an additional 4 h (until TLC indicated the complete consumption of B). The reaction mixture is diluted with ethyl acetate (100 mL) and quenched by addition of a saturated aqueous solution of ammonium chloride (30 mL). The organic layer is washed with brine (4 x 30 mL), dried over sodium sulfate, and evaporated under reduced pressure to give the crude product. This material is purified by flash column chromatography (eluting with 40% v/v ethyl acetate in hexanes) to give C as a yellow oil. 1H NMR (300 MHz, CDCl3): (50.86 (m, 2H, cyclopropyl CH2), 0.97 (m, 5H), 2.52 (s, 3H, SCH3), 3.00 (m, IH, cyclopropyl CH), 3.96 (q, JH-H = 7.0 Hz, 2H, CO2CH2CH3), 4.02 (apparent t, JH-F = 1.0 Hz, 3H, OCH3), 6.96 (m, IH, aromatic), 11.71 (s, IH). 19F(1H) NMR (282 MHz, CDCl3): £-149.9 (br, IF), -141.4 (br, IF), -135.7 (br, IF).
Step 4. Synthesis of ethyl l-cyclopropyl-6,7-difluoro-8-methoxy-2-(methylthio)-4-oxo-l,4- dihydroquinoline-3-carboxylate (D)
Sodium hydride (60% in mineral oil, 82 mg, 2.1 mmol) is added portionwise to a solution of C (760 mg, 1.95 mmol) in dimethylformamide (15 mL) at room temperature. The reaction mixture is heated at 80 0C for 3 d (until TLC indicates the complete consumption of B), cooled to room temperature, and quenched by addition of a saturated aqueous solution of ammonium chloride (10 mL). The mixture is extracted with ethyl acetate (3 x 50 mL). The combined organic extracts are washed with brine (4 x 30 mL), dried over sodium sulfate, and evaporated under reduced pressure to give crude D. This material is purified by flash column chromatography (eluting with 30% v/v ethyl acetate in hexanes) to D as a pale yellow oil.1H NMR (300 MHz, CDCl3): £0.73 (m, 2H, cyclopropyl CH2), 1.19 (m, 2H, cyclopropyl CH2), 1.38 (t, JH-H = 7.0 Hz, 3H, CO2CH2CH3), 2.66 (s, 3Η, SCH3), 3.74 (m, IH, cyclopropyl CH), 4.08 (d, JH-F = 2.5 Hz 3H, OCH3), 4.40 (q, JH_H = 7.0 Hz, 2H, CO2CH2CH3), 7.76 (dd, JH_F = 10.5 Hz, 8.5 Hz IH, aromatic). 19F(1H) NMR (282 MHz, CDCl3): £-146.8 (d, JF_F = 21.0 Hz, IF), - 137.7 (d, JF-F = 21.0 Hz, IF). LCMS mlz calcd for C17H17F2NO4S 369 ([M+]); found 370 ([M + H]+).
Step 5. Synthesis of ethyl l-cyclopropyl-6,7-difluoro-8-methoxy-2-(methylsulfinyl)-4-oxo-l,4- dihydroquinoline-3-carboxylate (E)
m-Chloroperoxybenzoic acid (<77%, 34 mg, 0.15 mmol) is added in one portion to a solution of D (50 mg, 0.14 mmol) in methylene chloride (3 mL) at room temperature. The reaction mixture is stirred for 1 h, diluted with ethyl acetate (20 mL), and washed with a 5% aqueous solution of sodium bicarbonate (2 x 10 mL). The organic layer is dried over sodium sulfate and evaporated under reduced pressure to give the crude product. This material is purified by preparative thin-layer chromatography (eluting with 10% v/v hexanes in ethyl acetate) to give pure E as a white solid. 1H NMR (300 MHz, CDCl3): £0.62 (m, IH, cyclopropyl CH2), 1.00 (m, IH, cyclopropyl CH2), 1.13 (m, IH, cyclopropyl CH2), 1.29 (m, IH, cyclopropyl CH2), 1.36 (t, JH_H = 7.5 Hz, 3H, CO2CH2CH3), 3.22 (s, 3Η, S(O)CH3), 3.85 (m, IH, cyclopropyl CH), 4.09 (d, JH-F = 2.5 Hz, 3H, OCH3), 4.37 (q, JH-H = 7.5 Hz, 2H, CO2CH2CH3), 7.75 (dd, JH-F = 10.0, 8.0 Hz, IH, aromatic). 19F(1H) NMR (282 MHz, CDCl3): £-145.2 (d, JF_F = 21.0 Hz, IF), -136.2 (d, JF_F = 21.0 Hz, IF). LCMS mlz calcd for C17H17F2NO5S 385 ([M+]); found 386 ([M + H]+).
Step 6. Synthesis of ethyl l-cyclopropyl-βJ-difluoro-l-mercaptoS-methoxy-^oxo-lA- dihydroquinoline-3-carboxylate (F).
Anhydrous sodium hydrogen sulfide (Alfa Aesar, 20 mg, 0.36 mmol) is added in one portion to a solution of DMF (6 mL) containing E (93 mg, 0.24 mmol) at room temperature. The resulting solution is heated at 40 0C for 2-3 h (until TLC indicated complete consumption of E) and allowed to cool to room temperature. The reaction mixture is quenched by addition of a 5% aqueous solution of hydrochloric acid (20 mL) and extracted with ethyl acetate (2 x 25 mL). The combined organic extracts are washed with brine (4 x 25 mL), dried over sodium sulfate, and evaporated to dryness under reduced pressure to give crude F in quantitative yield. This material is used directly in the next synthetic step to prevent its oxidative degradation. LCMS mlz calcd for C16H15F2NO4S 355 ([M+]); found 356 ([M + H]+) Step 7. Synthesis of9-cyclopropyl-6,7-difluoro-8-methoxyisothiazolo[5,4-b]quinoline- 3,4(2H,9H)-dione (3).
A solution of sodium bicarbonate (820 mg, 9.8 mmol) in water (14 mL) is added to a solution of F (348 mg, 0.98 mmol) in tetrahydrofuran (10 mL) at room temperature. Hydroxylamine-O-sulfonic acid (465 mg, 4.1 mmol) is added in one portion to this mixture. The reaction mixture is stirred at room temperature for ~3 h and quenched by addition of an aqueous solution of 5% hydrochloric acid (100 mL). The precipitate that formed is collected by filtration, washed with water (3 x 5 mL), and dried in vacuo to give 3 as a white solid. This product is of sufficient purity (>95% by 1H NMR spectroscopy) to use directly in the final amine-coupling step. 1HNMR (300 MHz, DMSO-J6): Jl.12 (m, 4H, cyclopropyl CH2), 3.85 (m, IH, cyclopropyl CH), 4.01 (d, JH-F= 1.5 Hz, 3H, OCH3), 7.85 (dd, JH_F = 11.0 Hz, 9.0 Hz, IH, aromatic). 19F(1H) NMR (282 MHz, DMSO-J6): £-146.4 (d, JF_F = 23.0 Hz, IF), -140.2 (d, JF_ F = 23.0 Hz, IF). LCMS mlz calcd for C14H10F2N2O3S 324 ([M*]); found 325 ([M + H]+).
REFERENCES
- Achillion Pharmaceuticals. About ACH-702. Available online: http://www.achillion.com/PL/pdf/04_ach_702_bg.pdf (accessed on 2 May 2013).
- Pucci, M.J.; Podos, S.D.; Thanassi, J.A.; Leggio, M.J.; Bradbury, B.J.; Deshpande, M. In vitro and in vivoprofiles of ACH-702, an isothiazoloquinolone, against bacterial pathogens. Antimicrob. Agents Chemother. 2011, 55, 2860–2871, doi:10.1128/AAC.01666-10.
- Achillion Pharmaceuticals, Inc. SEC filling form 10-Q quarterly report filed August 7, 2013. Available online: http://ir.achillion.com/secfiling.cfm?filingID=1193125–13–324297 (accessed on 28 September 2013).
- An efficient method for the synthesis of (R)-3-(1-amino-1-methylethyl)pyrrolidines for the antiinfective agent, PD 138312
Tetrahedron Asymmetry 1994, 5(7): 1131 - WO 2007014308
- WO 2008021491
WO2011031745A1 Sep 8, 2010 Mar 17, 2011 Achaogen, Inc. Antibacterial fluoroquinolone analogs HASHIMOTO, A. ET AL.: "Practical synthesis and molecular structure of a potent broad-spectrum antibacterial isothiazoloquinolone" ORG. PROCESS RESEARCH & DEVELOPMENT, vol. 11, 16 March 2007 (2007-03-16), pages 389-398, XP002465315 2 * WANG, Q. ET AL.: "Isothiazoloquinolones with Enhanced Antistaphylococcal Activities against Multidrug-Resistant Strains: Effects of Structural Modifications at the 6-, 7-, and 8-Positions" J. MED. CHEM., vol. 50, 2007, pages 199-210, XP002465316 WO2005019228A1 * Aug 4, 2004 Mar 3, 2005 Achillion Pharmaceuticals Inc Isothiazoloquinolones and related compounds as anti-infective agents WO2006118605A2 * Nov 10, 2005 Nov 9, 2006 Achillion Pharmaceuticals Inc 8a, 9-dihydro-4a-h-isothiazolo[5,4-b] quinoline-3, 4-diones and related compounds as anti-infective agents WO2007014308A1 * Jul 27, 2006 Feb 1, 2007 Achillion Pharmaceuticals Inc 8-methoxy-9h-isothiazolo[5,4-b]quinoline-3,4-diones and related compounds as anti-infective agents Citing Patent Filing date Publication date Applicant Title WO2008021491A2 * Aug 16, 2007 Feb 21, 2008 Achillion Pharmaceuticals Inc Method for synthesis of 8-alkoxy-9h-isothiazolo[5,4-b]quinoline-3,4-diones WO2011031745A1 Sep 8, 2010 Mar 17, 2011 Achaogen, Inc. Antibacterial fluoroquinolone analogs EP2488532A2 * Oct 15, 2010 Aug 22, 2012 Rib-X Pharmaceuticals, Inc. Antimicrobial compounds and methods of making and using the same US7902365 Aug 16, 2007 Mar 8, 2011 Achillion Pharmaceuticals, Inc. Method for synthesis of 8-alkoxy-9H-isothiazolo[5,4-B]quinoline-3,4-diones US8138346 Mar 4, 2011 Mar 20, 2012 Achillion Pharmaceuticals, Inc. Method for synthesis of 8-alkoxy-9H-isothiazolo[5,4-B]quinoline-3,4-diones











No comments:
Post a Comment