Pimobendan
CAS: 74150-27-9
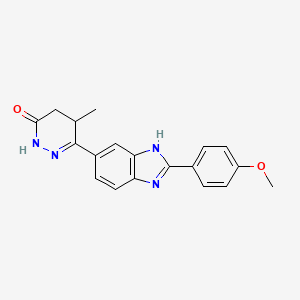
3-[2-(4-methoxyphenyl)-3H-benzimidazol-5-yl]-4-methyl-4,5-dihydro-1H-pyridazin-6-one
4,5-Dihydro-6-[2-(4-methoxyphenyl)-1H-benzimidazol-5-yl]-5-methyl-3(2H)-pyridazinone
UD-CG 115; UD-CG 115 BS; 2-(4-methoxyphenyl)-5(6)-(5-methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)benzimidazole
- 4,5-dihydro-6-(2-(4-methoxyphenyl)-1H-benzimidazole-5-yl)-5-methyl-3(2H)-pyridazinone
- pimobendan
- pimobendane
- UD CG 115
- UD-CG 115
- UD-CG115
MF: C19H18N4O2
MW: 334.37
Percent Composition: C 68.25%, H 5.43%, N 16.76%, O 9.57%
Derivative Type: Hydrochloride
CAS : 77469-98-8
MF: C19H18N4O2.HCl
MW: 370.83
Percent Composition: C 61.54%, H 5.16%, N 15.11%, O 8.63%, Cl 9.56%
Prop: Crystals from methanol and ethereal HCl, mp 311°(dec). LD50 orally in mice: ~600 mg/kg (Austel, 1982).
MP: mp 311°(dec)
Toxicity data: LD50 orally in mice: ~600 mg/kg (Austel, 1982)
Therap-Cat: Cardiotonic.
Pimobendan (INN is a veterinary medication manufactured by Boehringer Ingelheim under the trade names Vetmedin and Acardi) or pimobendane. It is a calcium sensitizer with positive inotropic and vasodilator effects. It is also a selective inhibitor ofphosphodiesterase III (PDE3).
Pimobendan is used in the management of heart failure in dogs, most commonly caused by myxomatous mitral valve disease (also known as endocardiosis), or dilated cardiomyopathy.[1] Research has shown that pimobendan increases survival time and improves quality of life in patients with congestive heart failure secondary to mitral valve disease when compared with benazepril, anangiotensin-converting-enzyme (ACE) inhibitor.[2] Under the trade name Acardi, it is available for human use in Japan.[3]
Mechanism of action
Pimobendan is a positive inotrope. It sensitizes and increases the binding efficiency of cardiac myofibril to the calcium ions that are already present without increasing the consumption of oxygen and energy. Pimobendan also causes peripheral vasodilation by inhibiting the function of phosphodiesterase III. This results in decreased pressure, translating into smaller cardiac preload andafterload (decreases the failing heart’s workload).
Pharmacokinetics
Pimobendan is absorbed rapidly when given via the oral route and has a bioavailability of 60-65%. It is metabolized into its active form by the liver. The half-life of pimobendan in the blood is 0.4 hours and the half-life of its metabolite is 2 hours. Elimination is by excretion in the bile and then feces. Pimobendan is 90–95% bound to plasma proteins in circulation. This has implications in patients suffering from low blood protein levels (hypoproteinemia/hypoalbuminemia) and with patients that are on concurrent therapies that are also highly protein bound.
Combinations
Pimobendan is often used in combination with three other drugs to palliate dogs with heart disease and reduce clinical signs of disease. These are:
- Furosemide, a diuretic, to reduce pulmonary edema. This can be given intravenously if the animal is in respiratory distress (6–8 mg/kg), and then titrated down to the minimum dose required orally.
- Spironolactone, an aldosterone antagonist. This has two actions, firstly, as a potassium-sparing diuretic, although its diuretic properties are small compared with those of furosemide. Secondly, it reduces aldosterone-mediated myocardial remodelling and fibrosis, slowing the progression of heart disease.
- An ACE inhibitor, often enalapril (trade name Enacard) or benazepril (Fortekor). These drugs inhibit the action of angiotensin-converting enzyme, producing a balanced vasodilation, along with other favourable effects.
Other drugs may also be used as required to manage certain arrhythmias that are often associated with heart disease.
Synthesis
Pimobendan can be synthesized beginning with anisoyl chloride.

Pimobendan synthesis:[4]
The cyclization of methyl 3- [4- (4-methoxybenzoylamino) -3-nitrobenzoyl] butyrate (I) with hydrazine hydrate in refluxing acetic acid gives 4,5-dihydro-6- [4- (4-methoxybenzoylamino) -3- nitrobenzoyl] -5-methylpyridazin-3 (2H) -one (II), which is reduced with H2 over Pd / C in ethanol yielding the corresponding amino derivative (III). Finally, this compound is cyclized in refluxing acetic acid.
References
- Gordon SG, Miller MW, Saunders AB (2006). “Pimobendan in heart failure therapy—a silver bullet?”. J Am Anim Hosp Assoc 42 (2): 90–3. PMID 16527909.
- Häggström J, Boswood A, O’Grady M, et al. (July 2008). “Effect of Pimobendan or Benazepril Hydrochloride on Survival Times in Dogs with Congestive Heart Failure Caused by Naturally Occurring Myxomatous Mitral Valve Disease: The QUEST Study”. J. Vet. Intern. Med. 22 (5): 1124–35. doi:10.1111/j.1939-1676.2008.0150.x. PMID 18638016.
- “Kusuri-no-Shiori Drug Information Sheet”. RAD-AR Council, Japan. April 2005. Retrieved 2008-08-06.
- Nicolas, C.; Verny, M.; Maurizis, J. C.; Payard, M.; Faurie, M. (1986). “Synthesis of 14C-bucromarone succinate and hydrochloride”. Journal of Labelled Compounds and Radiopharmaceuticals 23 (8): 837. doi:10.1002/jlcr.2580230806. edit
Further reading
- Lee JA, Allen DG (March 1990). “Calcium sensitisers”. BMJ 300 (6724): 551–2. doi:10.1136/bmj.300.6724.551. PMC 1662365. PMID 2108746.
External links
- Official Vetmedin Product Website
- UK Product Website
- Australia Product Website
- Russia Product Website
- Website about the QUEST study (reference 2) (http://www.questtrial.com)
- A Few Words About Pimobendan
 | |
| Systematic (IUPAC) name | |
|---|---|
| (RS)-6-[2-(4-methoxyphenyl)-1H-benzimidazol-5-yl]-5-methyl-4,5-dihydropyridazin-3(2H)-one | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status |
|
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 60 to 65% |
| Half-life | 0.4 hours |
| Excretion | In feces |
| Identifiers | |
| CAS number | 74150-27-9 |
| ATCvet code | QC01CE90 |
| PubChem | CID 4823 |
| ChemSpider | 4657 |
| UNII | 34AP3BBP9T |
| KEGG | D01133 |
| ChEMBL | CHEMBL24646 |
| Chemical data | |
| Formula | C19H18N4O2 |
| Mol. mass | 334.37 g/mol |
References: Positive inotropic agent. Prepn: V. Austel et al., DE 2837161; eidem, US 4361563 (1980, 1982 both to Thomae).
Cardiovascular effects: J. C. A. von Meel, Arzneim.-Forsch. 35, 284 (1985); P. D. Verdouw et al., Eur. J. Pharmacol.126, 21 (1986). Comparison of enantiomer activity: K. Fujino et al., J. Pharmacol. Exp. Ther. 247, 519 (1988).
Clinical trial in congestive heart failure: F. Hagemeijer, Am. Heart J. 122, 517 (1991); S. D. Katz et al., ibid. 123, 95 (1992).
No comments:
Post a Comment