THIS PAGE IS PART 1
PART 1...........http://drugsynthesisint.blogspot.in/p/gliptin-series.html
1.TRELAGLIPTIN
2.OMARIGLIPTIN
3.RETAGLIPTIN
4 CARMEGLIPTIN
5 IMIGLIPTIN
6 S SITAGLIPTIN
7 DENAGLIPTIN
8 DUTOGLIPTIN
SEE PART 2
9 TENELIGLIPTIN
10 GOSOGLIPTIN
11 EVOGLIPTIN
12 MELOGLIPTIN
13
14
PART 2 AT.......http://organicsynthesisinternational.blogspot.in/p/gliptin-series-22.html
will be updated with............
1 TRELAGLIPTIN

Trelagliptin succinate (SYR-472)
2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2, 4-dioxopyrimidin-1-yl]methyl]-4-fluorobenzonitrile; butanedioic acid
2-[6-[3(R)-Aminopiperidin-1-yl]-3-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-ylmethyl]-4-fluorobenzonitrile
2- [ [6- [ (3R) -3-amino-l-piperidinyl] -3, 4-dihydro-3- methyl-2, 4-dioxo-l (2H) -pyrimidinyl]methyl] -4-fluorobenzonitrile
succinic acid salt of 2-[6-(3-amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile
Sponsor/Developer: Takeda Pharmaceuticals and Furiex Pharmaceuticals
Mechanism of action: DPP-4 inhibitor
865759-25-7 cas FREE BASE
1029877-94-8 succinate
- SYR 111472 succinate
- SYR 472
- Syr-472
- Syr111472 succinate
- Trelagliptin succinate
- UNII-4118932Z90
- clinical trials....http://clinicaltrials.gov/search/intervention=SYR+472
Trelagliptin-succinate M. Wt: 475.47
Trelagliptin-succinate Formula: C22H26FN5O6
Trelagliptin-succinate Formula: C22H26FN5O6
SYR-472 is an oral dipeptidyl peptidase IV inhibitor originated by Takeda. It is in phase III clinical trials for the treatment of type 2 diabetes.
- Diabetes affects 25.8 million people of all ages, or roughly 8.3 percent of the U.S. population.
- The World Health Organization predicts that there will be 366 million people worldwide affected by diabetes by the year 2030.
- The advent of trelagliptin succinate, a unique once weekly medication for patients with type 2 Diabetes is now the focus of clinical trials and exciting research and development.
- Phase III clinical trials of trelagliptin succinate commenced in September 2011, and are estimated to be complete by the second half of 2013.
TRELAGLIPTIN (SYR-472)
Trelagliptin is a novel DPP-4 inhibitor that is being developed by Takeda. In contrast to alogliplitin, which is once a day, trelagliptin is a once-weekly oral agent which should provide patients with a convenient therapeutic alternative and has the potential to improve compliance. Takeda has commenced Phase III trials of trelagliptin in Japan for the treatment of Type 2 diabetes.

Indication (Phase): Japan—Once-weekly oral treatment for type 2 diabetes (Phase III; study expected to be completed in second half of 2013)
Compound I, A, TRELAGLIPTIN which has the formula:
is a DPP-IV inhibitor that is described in U.S. patent application Ser. No. 11/080,992 filed Mar. 15, 2005 (see Compound 34). Its dosing, administration and biological activities are described in U.S. patent application Ser. No. 11/531,671 filed Sep. 13, 2006. U.S. patent application Ser. No. 11/080,992 and Ser. No. 11/531,671 are incorporated herein by reference in their entirety.
Dipeptidyl peptidase IV (IUBMB Enzyme Nomenclature EC.3.4.14.5) (referred herein as “DPP-IV”) is a type II membrane protein and a non-classical serine aminodipeptidase that removes Xaa-Pro dipeptides from the amino terminus (N-terminus) of polypeptides and proteins. DPP-IV is constitutively expressed on epithelial and endothelial cells of a variety of different tissues (e.g., intestine, liver, lung, kidney and placenta), and is also found in body fluids. DPP-IV is also expressed on circulating T-lymphocytes and has been shown to be synonymous with the cell-surface antigen, CD-26. DPP-IV has been implicated in a number of human disease states, including, but are not limit to, diabetes, particularly type II diabetes mellitus, diabetic dislipidemia, conditions of impaired glucose tolerance (IGT), conditions of impaired fasting plasma glucose (IFG), metabolic acidosis, ketosis, appetite regulation and obesity; autoimmune diseases such as inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis; AIDS; and cancers.
DPP-IV inhibitors are believed to be useful agents for the prevention, delay of progression, and/or treatment of conditions mediated by DPP-IV.
Compound (A) or a salt thereof has been reported as an inhibitor of dipeptidyl peptidase (DPP-IV) , which is an enzyme that decomposes glucagon-like peptide-1 (GLP-1) , a hormone increasing insulin secretion (patent document 1) .
In addition, a method including administering 1 - 250 mg of compound (A) or a salt thereof to a patient once per week (patent documents 2, 3), crystal polymorphs of compound (A) (patent documents 4, 5) , and a preparation of compound (A)
(patent documents 6, 7) have also been reported. Compound (A) and a salt thereof are recommended for oral administration in view of the easiness of self-administration, and a tablet, particularly a tablet in the dosage form for administration once per week, is desired. [0006]
The dosage form of once per week is expected to improve drug compliance of patients, whereas it requires supply of compound (A) or a salt thereof to patients in a high dose as compared to, for example, the dosage form of once per day. Since a solid preparation containing compound (A) or a salt thereof in a high dose increases its size, it may conversely degrade the drug compliance for patients, particularly infants and elderly patients having difficulty in swallowing
..........................
SYNTHESIS
Compound 34 IS TRELAGLIPTIN
4-Fluoro-2-methylbenzonitrile (31).
A mixture of 2-bromo-5-fluorotoluene (3.5 g, 18.5 mmol) and CuCN (2 g, 22 mmol) in DMF (100 mL) was refluxed for 24 hours. The reaction was diluted with water and extracted with hexane. The organics were dried over MgSO4 and the solvent removed to give product 31 (yield 60%). 1H-NMR (400 MHz, CDCl3): δ 7.60 (dd, J=5.6, 8.8 Hz, 1H), 6.93-7.06 (m, 2H), 2.55 (s, 3H).
2-Bromomethyl-4-fluorobenzonitrile (32).
A mixture of 4-fluoro-2-methylbenzonitrile (2 g, 14.8 mmol), NBS (2.64 g, 15 mmol) and AIBN (100 mg) in CCl4 was refluxed under nitrogen for 2 hours. The reaction was cooled to room temperature. The solid was removed by filtration. The organic solution was concentrated to give crude product as an oil, which was used in the next step without further purification. 1H-NMR (400 MHz, CDCl3): δ 7.68 (dd, J=5.2, 8.4 Hz, 1H), 7.28 (dd, J=2.4, 8.8 Hz, 1H), 7.12 (m, 1H), 4.6 (s, 2H).
Alternatively, 32 was made as follows.
4-Fluoro-2-methylbenzonitrile (1 kg) in DCE (2 L) was treated with AIBN (122 g) and heated to 75° C. A suspension of DBH (353 g) in DCE (500 mL) was added at 75° C. portionwise over 20 minutes. This operation was repeated 5 more times over 2.5 hours. The mixture was then stirred for one additional hour and optionally monitored for completion by, for example, measuring the amount of residual benzonitrile using HPLC. Additional AIBN (e.g., 12.5 g) was optionally added to move the reaction toward completion. Heating was stopped and the mixture was allowed to cool overnight. N,N-diisopropylethylamine (1.3 L) was added (at <10° C. over 1.5 hours) and then diethyl phosphite (1.9 L) was added (at <20° C. over 30 min). The mixture was then stirred for 30 minutes or until completion. The mixture was then washed with 1% sodium metabisulfite solution (5 L) and purified with water (5 L). The organic phase was concentrated under vacuum to afford 32 as a dark brown oil (3328 g), which was used without further purification (purity was 97% (AUC)).
2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile (33).
A mixture of crude 3-methyl-6-chlorouracil (0.6 g, 3.8 mmol), 2-bromomethyl-4-fluorobenzonitrile (0.86 g, 4 mmol) and K2CO3 (0.5 g, 4 mmol) in DMSO (10 mL) was stirred at 60° C. for 2 hours. The reaction was diluted with water and extracted with EtOAc. The organics were dried over MgSO4 and the solvent removed. The residue was purified by column chromatography. 0.66 g of the product was obtained (yield: 60%). 1H-NMR (400 MHz, CDCl3): δ 7.73 (dd, J=7.2, 8.4 Hz, 1H), 7.26 (d, J=4.0 Hz, 1H), 7.11-7.17 (m, 1H), 6.94 (dd, J=2.0, 9.0 Hz, 1H), 6.034 (s, 2H), 3.39 (s, 3H). MS (ES) [m+H] calc'd for C13H9ClFN3O2, 293.68; found 293.68.
Alternatively, 33 was made as follows.
To a solution of 6-chloro-3-methyluracil (750 g) and N,N-diisopropylethylamine (998 mL) in NMP (3 L) was added (at <30° C. over 25 min) a solution of 32 (2963 g crude material containing 1300 g of 32 in 3 L of toluene). The mixture was then heated at 60° C. for 2 hours or until completion (as determined, for example, by HPLC). Heating was then stopped and the mixture was allowed to cool overnight. Purified water (3.8 L) was added, and the resultant slurry was stirred at ambient temperature for 1 hour and at <5° C. for one hour. The mixture was then filtered under vacuum and the wet cake was washed with IPA (2×2.25 L). The material was then dried in a vacuum oven at 40±5° C. for 16 or more hours to afford 33 as a tan solid (>85% yield; purity was >99% (AUC)).
TFAsalt OF TRELAGLIPTIN
2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile (34).
2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile (300 mg, 1.0 mmol), (R)-3-amino-piperidine dihydrochloride (266 mg, 1.5 mmol) and sodium bicarbonate (500 mg, 5.4 mmol) were stirred in a sealed tube in EtOH (3 mL) at 100° C. for 2 hrs. The final compound was obtained as TFA salt after HPLC purification. 1H-NMR (400 MHz, CD3OD): δ. 7.77-7.84 (m, 1H), 7.16-7.27 (m, 2H), 5.46 (s, 1H), 5.17-5.34 (ABq, 2H, J 35.2, 15.6 Hz), 3.33-3.47 (m, 2H), 3.22 (s, 3H), 2.98-3.08 (m, 1H), 2.67-2.92 (m, 2H), 2.07-2.17 (m, 1H), 1.82-1.92 (m, 1H), 1.51-1.79 (m, 2H). MS (ES) [m+H] calc'd for C18H20FN5O2, 357.38; found, 357.38.
FREE BASE NOF TRELAGLIPTIN
Alternatively, the free base of 34 was prepared as follows. A mixture of 33 (1212 g), IPA (10.8 L), (R)-3-amino-piperidine dihydrochloride (785 g), purified water (78 mL) and potassium carbonate (2.5 kg, powder, 325 mesh) was heated at 60° C. until completion (e.g., for >20 hours) as determined, for example, by HPLC. Acetonitrile (3.6 L) was then added at 60° C. and the mixture was allowed to cool to <25° C. The resultant slurry was filtered under vacuum and the filter cake was washed with acetonitrile (2×3.6 L). The filtrate was concentrated at 45° C. under vacuum (for >3 hours) to afford 2.6 kg of the free base of 34.
HCL salt OF TRELAGLIPTIN
The HCl salt of 34 was prepared from the TFA salt as follows. The TFA salt (34) was suspended in DCM, and then washed with saturated Na2CO3. The organic layer was dried and removed in vacuo. The residue was dissolved in acetonitrile and HCl in dioxane (1.5 eq.) was added at 0° C. The HCl salt was obtained after removing the solvent. 1H-NMR (400 MHz, CD3OD): δ. 7.77-7.84 (m, 1H), 7.12-7.26 (m, 2H), 5.47 (s, 1H), 5.21-5.32 (ABq, 2H, J=32.0, 16.0 Hz), 3.35-3.5 (m, 2H), 3.22 (s, 3H), 3.01-3.1 (m, 1H), 2.69-2.93 (m, 2H), 2.07-2.17 (m, 1H), 1.83-1.93 (m, 1H), 1.55-1.80 (m, 2H). MS (ES) [m+H] calc'd for C18H20FN5O2, 357.38; found, 357.38.
Alternatively, the HCl salt was prepared from the free base as follows. To a solution of free base in CH2Cl2 (12 L) was added (at <35° C. over 18 minutes) 2 M hydrochloric acid (3.1 L). The slurry was stirred for 1 hour and then filtered. The wet cake was washed with CH2Cl2 (3.6 L) and then THF (4.8 L). The wet cake was then slurried in THF (4.8 L) for one hour and then filtered. The filter cake was again washed with THF (4.8 L). The material was then dried in a vacuum oven at 50° C. (with a nitrogen bleed) until a constant weight (e.g., >26 hours) to afford 34 as the HCl salt as a white solid (1423 g, >85% yield).
Succinate salt OF TRELAGLIPTIN

The succinate salt of 34 was prepared from the HCl salt as follows. To a mixture of the HCl salt of 34 (1414 g), CH2Cl2 (7 L) and purified water (14 L) was added 50% NaOH solution (212 mL) until the pH of the mixture was >12. The biphasic mixture was stirred for 30 min and the organic layer was separated. The aqueous layer was extracted with CH2Cl2 (5.7 L) and the combined organic layers were washed with purified water (6 L). The organic layer was then passed through an in-line filter and concentrated under vacuum at 30° C. over three hours to afford the free base as an off-white solid. The free base was slurried in prefiltered THF (15 L) and prefiltered IPA (5.5 L). The mixture was then heated at 60° C. until complete dissolution of the free base was observed. A prefiltered solution of succinic acid (446 g) in THF (7 L) was added (over 23 min) while maintaining the mixture temperature at >57° C. After stirring at 60° C. for 15 min, the heat was turned off, the material was allowed to cool, and the slurry was stirred for 12 hours at 25±5° C. The material was filtered under vacuum and the wet cake was washed with prefiltered IPA (2×4.2 L). The material was then dried in a vacuum oven at 70±5° C. (with a nitrogen bleed) for >80 hours to afford the succinate salt of 34 as a white solid (1546 g, >90% yield).
The product was also converted to a variety of corresponding acid addition salts. Specifically, the benzonitrile product (approximately 10 mg) in a solution of MeOH (1 mL) was treated with various acids (1.05 equivalents). The solutions were allowed to stand for three days open to the air. If a precipitate formed, the mixture was filtered and the salt dried. If no solid formed, the mixture was concentrated in vacuo and the residue isolated. In this way, salts of 34 were prepared from the following acids: benzoic, p-toluenesulfonic, succinic, R-(−)-Mandelic and benzenesulfonic. The succinate was found to be crystalline as determined by x-ray powder diffraction analysis.
Methanesulfonate salt
In addition, the methanesulfonate salt was prepared as follows. A 10.5 g aliquot of the benzonitrile product was mixed with 400 mL of isopropylacetate. The slurry was heated to 75° C. and filtered through #3 Whatman filter paper. The solution was heated back to 75° C. and a 1M solution of methanesulfonic acid (30.84 mL) was added slowly over 10 minutes while stirring. The suspension was cooled to room temperature at a rate of about 20° C./hr. After 1 hr at room temperature, the solid was filtered and dried in an oven overnight to obtain the methanesulfonate salt.
..............................
FORMULATION
COMPD A IS TRELAGLIPTIN
Examples (Comparative Example IA)
Succinate of compound (A) (26.6 mg) was weighed in a glass bottle and used as Comparative Example IA. (Comparative Example 2A)
The succinate of compound (A) and microcrystalline cellulose were uniformly mixed in a mortar at a ratio of 1:10, and the mixture (226.6 mg) was weighed in a glass bottle and used as Comparative Example 2A. (Comparative Example 3A)
The succinate of compound (A) and corn starch were uniformly mixed in a mortar at a ratio of 1:5, and the mixture (126.6 mg) was weighed in a glass bottle and used as Comparative Example 3A. (Example IA) Succinate of compound (A) , mannitol and corn starch according to the formulation of Table IA were uniformly mixed in a fluid bed granulator (LAB-I, POWREX CORPORATION) , and the mixture was granulated by spraying an aqueous solution of dissolved hypromellose 2910, and dried therein. The obtained granules were passed through a sieve -(16M) to give milled granules. To the milled granules were added croscarmellose sodium, microcrystalline cellulose and magnesium stearate, and they were mixed in a bag to give granules for tableting. The granules were punched by a rotary tableting machine (Correct 19K, Kikusui Seisakusho, Ltd.) with a 6.5 mmφ punch to give a plain tablet weighting 121 mg. On the other hand, titanium oxide, yellow ferric oxide and talc were dispersed in a hypromellose 2910 aqueous solution to prepare a film coating liquid. The aforementioned coating liquid was sprayed onto the above-mentioned plain tablet in a film coating machine (Hicoater HCP-75, Freund Corporation), to give 2500 film- coated tablets containing 3.125 mg of compound (A) (free form) per tablet. Table IA
.............................
POLYMORPHS AND SYNTHESIS
FORM A
Form A may be prepared by crystallization from the various solvents and under the various crystallization conditions used during the polymorph screen (e.g., fast and slow evaporation, cooling of saturated solutions, slurries, and solvent/antisolvent additions). Tables B and C of Example 3 summarize the procedures by which Form A was prepared. For example, Form A was obtained by room temperature slurry of an excess amount of Compound I in acetone, acetonitrile, dichloromethane, 1,4-dioxane, diethyl ether, hexane, methanol, isopropanol, water, ethylacetate, tetrahydrofuran, toluene, or other like solvents on a rotating wheel for approximately 5 or 7 days. The solids were collected by vacuum filtration, and air dried in the hood. Also, Form A was precipitated from a methanol solution of Compound I by slow evaporation (SE).
[0091] Form A was characterized by XRPD, TGA, hot stage microscopy, IR, Raman spectroscopy, solution 1H-NMR, and solid state 13C-NMR.
[0092] Figure 1 shows a characteristic XRPD spectrum (CuKa, λ=1.5418A) of Form A. The XRPD pattern confirmed that Form A was crystalline. Major X-Ray diffraction lines expressed in °2Θ and their relative intensities are summarized in Table 1.
Table 1. Characteristic XRPD Peaks (CuKa) of Form A
8. Amorphous Form
[0137] The Amorphous Form of Compound I was prepared by lyophilization of an aqueous solution of Compound I (Example 10). The residue material was characterized by XRPD and the resulting XRPD spectrum displayed in Figure 26. The XRPD spectrum shows a broad halo with no specific peaks present, which confirms that the material is amorphous. The material was further characterized by TGA, DSC, hot stage microscopy, and moisture sorption analysis.
Table A. Approximate Solubilities of Compound I
Compound I having the formula
POLYMORPH SCREEN
Crystallization Experiments of Compound I from Solvents
a) FE = fast evaporation; SE = slow evaporation; RT = room temperature; SC = slow cool;CC = crash cool, MB = moisture sorption/desorption analysis b) qty = quantity; PO = preferred orientation
..............................
SYNTHESIS
EXAMPLES
1. Preparation of 2-[6-(3-Amino-piperidin-l-yl)-3-methyl-2,4-dioxo-3,4-dihydro- 2H-pyrimidin-l-ylmethyl]-4-fluoro-benzonitrile and pharmaceutically acceptable salts
4-Fluoro-2-methylbenzonitrile (3)
[0166] A mixture of 2-bromo-5fluorotoluene ( 2) (3.5 g, 18.5 mmol) and CuCN (2 g, 22 mmol) in DMF (100 mL) was re fluxed for 24 hours. The reaction was diluted with water and extracted with hexane. The organics were dried over MgSO4 and the solvent removed to give product 3 (yield 60%). 1H-NMR (400 MHz, CDCl3): δ 7.60 (dd, J=5.6, 8.8 Hz, IH), 6.93-7.06 (m, 2H), 2.55 (s, 3H). 2-Bromomethyl-4-fluorobenzonitrile (4)
[0167] A mixture of 4-fluoro-2-methylbenzonitrile (3) (2 g, 14.8 mmol), NBS (2.64 g, 15 mmol) and AIBN (100 mg) in CCl4 was refluxed under nitrogen for 2 hours. The reaction was cooled to room temperature. The solid was removed by filtration. The organic solution was concentrated to give crude product as an oil, which was used in the next step without further purification.1H-NMR (400 MHz, CDCl3): δ 7.68 (dd, J= 5.2, 8.4 Hz, IH), 7.28 (dd, J= 2.4, 8.8 Hz, IH), 7.12 (m, IH), 4.6 (s, 2H).
2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-l-ylmethyl)-4-fluoro- benzonitrile (6)
[0168] A mixture of crude 3-methyl-6-chlorouracil (5) (0.6 g, 3.8 mmol), 2- Bromomethyl-4-fluorobenzonitrile (0.86 g, 4 mmol) and K2CO3 (0.5 g, 4 mmol) in DMSO
(10 mL) was stirred at 60 C for 2 hours. The reaction was diluted with water and extracted with EtOAc. The organics were dried over MgSO4 and the solvent removed. The residue was purified by column chromatography. 0.66 g of the product was obtained (yield: 60%). 1H-NMR (400 MHz, CDCl3): δ 7.73 (dd, 1=12, 8.4Hz, IH), 7.26 (d, J- 4.0Hz, IH), 7.11-7.17 (m, IH), 6.94 (dd, J=2.0, 9.0 Hz, IH), 6.034 (s, 2H), 3.39 (s, 3H). MS (ES) [m+H] calc'd for Ci3H9ClFN3O2, 293.68; found 293.68.
2-[6-(3-Amino-piperidin-l-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-l- ylmethyl]-4-fluoro-benzonitrile, TFA salt (1) (TFA salt of Compound I)
[0169] 2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-l-ylmethyl)-4- fluoro-benzonitrile (5) (300 mg, 1.0 mmol), (i?)-3-amino-piperidine dihydrochloride (266 mg, 1.5 mmol) and sodium bicarbonate (500 mg, 5.4 mmol) were stirred in a sealed tube in EtOH (3 mL) at 100 0C for 2 hrs. The final compound was obtained as a TFA salt after HPLC purification. 1H-NMR (400 MHz, CD3OD): δ. 7.77-7.84 (m, IH), 7.16-7.27 (m, 2H), 5.46 (s, IH), 5.17-5.34 (ABq, 2H, J = 35.2, 15.6 Hz), 3.33-3.47 (m, 2H), 3.22 (s, 3H), 2.98-3.08 (m, IH), 2.67-2.92 (m, 2H), 2.07-2.17 (m, IH), 1.82-1.92 (m, IH), 1.51-1.79 (m, 2H). MS (ES) [m+H] calc'd for Ci8H20FN5O2, 357.38; found, 357.38.
2-[6-(3-Amino-piperidin-l-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-l- ylmethyl]-4-fluoro-benzonitrile, HCl salt
[0170] The TFA salt of Compound I was suspended in DCM, and then washed with saturated Na2CO3. The organic layer was dried and removed in vacuo. The residue was dissolved in acetonitrile and HCl in dioxane (1.5 eq.) was added at 0 C. The HCl salt was obtained after removing the solvent. 1H-NMR (400 MHz, CD3OD): δ. 7.77-7.84 (m, IH), 7.12-7.26 (m, 2H), 5.47 (s, IH), 5.21-5.32 (ABq, 2H, J = 32.0, 16.0 Hz), 3.35-3.5 (m, 2H), 3.22 (s, 3H), 3.01-3.1 (m, IH), 2.69-2.93 (m, 2H), 2.07-2.17 (m, IH), 1.83-1.93 (m, IH), 1.55-1.80 (m, 2H). MS (ES) [m+H] calc'd for Ci8H20FN5O2, 357.38; found, 357.38.
General procedure for the preparation of salts of Compound I.
[0171] The benzonitrile product may be isolated as the free base if desired, but preferably, the product may be further converted to a corresponding acid addition salt. Specifically, the benzonitrile product (approximately 10 mg) in a solution of MeOH (1 mL) was treated with various acids (1.05 equivalents). The solutions were allowed to stand for three days open to the air. If a precipitate formed, the mixture was filtered and the salt dried. If no solid formed, the mixture was concentrated in vacuo and the residue isolated. In this way, salts of Compound I were prepared from the following acids: benzoic, p-toluenesulfonic, succinic, R-(-)-Mandelic and benzenesulfonic. [0172] The isolation and/or purification steps of the intermediate compounds in the above described process may optionally be avoided if the intermediates from the reaction mixture are obtained as relatively pure compounds and the by-products or impurities of the reaction mixture do not interfere with the subsequent reaction steps. Where feasible, one or more isolation steps may be eliminated to provide shorter processing times, and the elimination of further processing may also afford higher overall reaction yields.
.......................
TABLET
2. Exemplary formulations comprising succinate salt of 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile
Provided are examples of tablet formulations that may be used to administer succinate salt of 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile (Succinate salt of Compound I) according to the present invention. It is noted that the formulations provided herein may be varied as is known in the art.
The exemplary tablet formulations are as follows:
| 12.5 mg of Compound I (weight of free base form) per tablet | ||||
| Core Tablet Formulation | ||||
| (1) | 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4- | 17.0 | mg | |
| dioxo-3,4-dihydro-2H-pyrimidin-1- | ||||
| ylmethyl]-4-fluoro-benzonitrile (succinate salt) | ||||
| (2) | Lactose Monohydrate, NF, Ph, Eur | 224.6 | mg | |
| (FOREMOST 316 FAST FLO) | ||||
| (3) | Microcrystalline Cellulose, NF, Ph, Eur | 120.1 | mg | |
| (AVICEL PH 102) | ||||
| (4) | Croscarmellose Sodium, NF, Ph, Eur | 32.0 | mg | |
| (AC-DO-SOL) | ||||
| (5) | Colloidal Silicon Dioxide, NF, Ph, Eur | 3.2 | mg | |
| (CAB-O-SIL M-5P) | ||||
| (6) | Magnesium Stearate, NF, Ph, Eur | 3.2 | mg | |
| (MALLINCKRODT, Non-bovine Hyqual) | ||||
| TOTAL | 400.0 | mg | ||
| (per tablet) | ||||
..............
POLYMORPHS AND SYNTHESIS
EXAMPLES Example 1 Preparation of 2-[6-(3-amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile succinate (Compound I)
Compound I may be prepared by the follow synthetic route (Scheme 1)
A. Preparation of 4-fluoro-2-methylbenzonitrile (Compound B)
Compound B was prepared by refluxing a mixture of 2-bromo-5-fluoro-toluene (Compound A) (3.5 g, 18.5 mmol) and CuCN (2 g, 22 mmol) in DMF (100 mL) for 24 hours. The reaction was diluted with water and extracted with hexane. The organics were dried over MgSO4 and the solvent removed to give product B (yield 60%). 1H-NMR (400 MHz, CDCl3): δ 7.60 (dd, J=5.6, 8.8 Hz, 1H), 6.93-7.06 (m, 2H), 2.55 (s, 3H).
B. Preparation of 2-bromomethyl-4-fluorobenzonitrile (Compound C)
Compound C was prepared by refluxing a mixture of 4-fluoro-2-methylbenzonitrile (Compound B) (2 g, 14.8 mmol), N-bromosuccinimide (NBS) (2.64 g, 15 mmol) and azo-bis-isobutyronitrile (AIBN) (100 mg) in CCl4 under nitrogen for 2 hours. The reaction was cooled to room temperature. The solid was removed by filtration. The organic solution was concentrated to give the crude product the form of an oil, which was used in the next step without further purification. 1H-NMR (400 MHz, CDCl3): δ 7.68 (dd, J=5.2, 8.4 Hz, 1H), 7.28 (dd, J=2.4, 8.8 Hz, 1H), 7.12 (m, 1H), 4.6 (s, 2H).
C. Preparation of 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile (Compound D)
Compound E was prepared by stirring a mixture of crude 3-methyl-6-chlorouracil D (0.6 g, 3.8 mmol), 2-bromomethyl-4-fluorobenzonitrile (0.86 g, 4 mmol) and K2CO3 (0.5 g, 4 mmol) in DMSO (10 mL) at 60° C. for 2 hours. The reaction was diluted with water and extracted with EtOAc. The organics were dried over MgSO4 and the solvent removed. The residue was purified by column chromatography. 0.66 g of the product was obtained (yield: 60%). 1H-NMR (400 MHz, CDCl3): δ 7.73 (dd, J=7.2, 8.4 Hz, 1H), 7.26 (d, J=4.0 Hz, 1H), 7.11-7.17 (m, 1H), 6.94 (dd, J=2.0, 9.0 Hz, 1H), 6.034 (s, 2H), 3.39 (s, 3H). MS (ES) [m+H] calc'd for C13H9ClFN3O2, 293.68; found 293.68.
D. Preparation of 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile (Compound F)
Compound F was prepared by mixing and stirring 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile (Compound E) (300 mg, 1.0 mmol), (R)-3-amino-piperidine dihydrochloride (266 mg, 1.5 mmol) and sodium bicarbonate (500 mg, 5.4 mmol) in a sealed tube in EtOH (3 mL) at 100° C. for 2 hrs. The final compound was obtained as trifluoroacetate (TFA) salt after HPLC purification. 1H-NMR (400 MHz, CD3OD): δ. 7.77-7.84 (m, 1H), 7.16-7.27 (m, 2H), 5.46 (s, 1H), 5.17-5.34 (ABq, 2H, J=35.2, 15.6 Hz), 3.33-3.47 (m, 2H), 3.22 (s, 3H), 2.98-3.08 (m, 1H), 2.67-2.92 (m, 2H), 2.07-2.17 (m, 1H), 1.82-1.92 (m, 1H), 1.51-1.79 (m, 2H). MS (ES) [m+H] calc'd for C18H20FN5O2, 357.38; found, 357.38.
E. Preparation of Compound I: the succinic acid salt of 2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-4-fluoro-benzonitrile
The TFA salt prepared in the above step (Example 1, Step D) was suspended in DCM, and then washed with saturated Na2CO3. The organic layer was dried and removed in vacuo. The benzonitrile product (approximately 10 mg) was dissolved in MeOH (1 mL) and to which succinic acid in THF (1.05 equivalents) was added. The solutions were allowed to stand for three days open to the air. If a precipitate formed, the solid was collected by filtration. If no solid formed, the mixture was concentrated in vacuo, and the succinate salt was obtained after removing the solvent.
SUCCINATE SALT OF TRELAGLIPTIN
1H-NMR (400 MHz, CD3OD): δ. 7.77-7.84 (m, 1H), 7.12-7.26 (m, 2H), 5.47 (s, 1H), 5.21-5.32 (ABq, 2H, J=32.0, 16.0 Hz), 3.35-3.5 (m, 2H), 3.22 (s, 3H), 3.01-3.1 (m, 1H), 2.69-2.93 (m, 2H), 2.07-2.17 (m, 1H), 1.83-1.93 (m, 1H), 1.55-1.80 (m, 2H). MS (ES) [m+H] calc'd for C18H20FN5O2, 357.38; found, 357.38.
Compound I such prepared was found to be crystalline as determined by x-ray powder diffraction analysis (FIG. 1). The crystal material was designated Form A.
...............
patents
1. US 2013172377
2. WO 2011013639
3. WO 2009099172
4.WO 2009099171
5. WO 2008114807
6.WO 2008114800
7. WO 2008033851
8. WO 2007074884
9WO 2007035629
patent document 1: US2005/0261271
patent document 2: US2007/0060530
patent document 3: US2008/0287476
patent document 4: US2008/0227798
patent document 5: US2008/0280931
patent document 6: WO2008/114800
patent document 7: WO2011/013639
| US7906523 * | Oct 30, 2007 | Mar 15, 2011 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US8084605 * | Nov 29, 2007 | Dec 27, 2011 | Kelly Ron C | Polymorphs of succinate salt of 2-[6-(3-amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethy]-4-fluor-benzonitrile and methods of use therefor |
| US8188275 * | Oct 30, 2007 | May 29, 2012 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US8222411 * | Sep 15, 2006 | Jul 17, 2012 | Takeda Pharmaceutical Company Limited | Dipeptidyl peptidase inhibitors |
| US20090275750 * | Sep 15, 2006 | Nov 5, 2009 | Jun Feng | Dipeptidyl peptidase inhibitors |
| WO2013183784A1 | Jun 4, 2013 | Dec 12, 2013 | Takeda Pharmaceutical Company Limited | Solid preparation |
| US20080227798 * | Nov 29, 2007 | Sep 18, 2008 | Kelly Ron C | Polymorphs of succinate salt of 2-[6-(3-amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2h-pyrimidin-1-ylmethy]-4-fluor-benzonitrile and methods of use therefor |
| US20120197018 * | Feb 15, 2012 | Aug 2, 2012 | Kelly Ron C | Polymorphs of succinate salt of 2-[6-(3-amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2h-pyrimidin-1-ylmethy]-4-fluor-benzonitrile and methods of use therefor |
| WO2007033265A1 * | Sep 13, 2006 | Mar 22, 2007 | Takeda Pharmaceutical | Dipeptidyl peptidase inhibitors for treating diabetis |
| WO2007033266A2 * | Sep 13, 2006 | Mar 22, 2007 | Takeda Pharmaceutical | Dipeptidyl peptidase inhibitors for treating diabetis |
| WO2007033350A1 * | Sep 13, 2006 | Mar 22, 2007 | Takeda Pharmaceutical | Dipeptidyl peptidase inhibitors for treating diabetes |
| EP1586571A1 * | Dec 21, 2004 | Oct 19, 2005 | Takeda San Diego, Inc. | Dipeptidyl peptidase inhibitors |
2 OMARIGLIPTIN
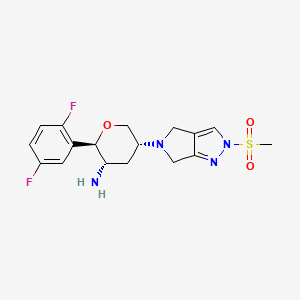
OMARIGLIPTIN. MK 3102
1226781-44-7
(2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine
(2R,3S,5R)-2-(2,5-difluorophenyl)-5-(2-methylsulfonyl-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yl)oxan-3-amine
1226781-44-7, Omarigliptin [USAN], Omarigliptin (USAN/INN), SureCN827590, UNII-CVP59Q4JE1, CHEMBL2105762, MK-3102, PB39113
Molecular Formula: C17H20F2N4O3S Molecular Weight: 398.427506
IN PHASE 3
……………………………..
Example 1
(2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amineStep A: tert-Butyl {(2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5 (4H)-yl]tetrahydro-2H-pyran-3-yl}carbamate
A mixture of Intermediate 2 (26.3 g, 80 mmol) and 2-(methylsulfonyl)-2,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole (Intermediate 5) (15.07 g, 80 mmol) in anhydrous methanol (1.5 L) was stirred at room temperature for 2 h. To the resulting white suspension was added decaborane (2.95 g, 24.15 mmol) and the mixture was stirred at room temperature overnight. Methanol was removed and the residue was purified on two 65i Biotage™ columns eluting with 5-50% ethyl acetate in dichloromethane to afford the title compound as a white solid. LC-MS: 499.10 (M+1).
Step B: (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine
Removal of the BOC group in the product from Step A (13.78 g, 27.67 mmol) was accomplished with trifluoroacetic acid (100 ml) in dichloromethane (200 mL) at room temperature. After stirring for 2 h, the reaction was concentrated and neutralized with 25% MeOH and 2.5% ammonium hydroxide in dichloromethane. Solvents were removed under reduced pressure and the resulting crude material was purified on a 65i Biotage™ column eluting with 1.25-5% MeOH and 0.125-0.5% ammonium hydroxide in dichloromethane. The isolated material was further purified by recrystallization from 5:1 EtOAc/CH2Cl2 at 60° C. The crystalline product was washed with cold 2:1EtOAc/hexanes to give the title compound as a light brown solid. 1H NMR (500 MHz, CD3OD): 1.71 (q, 1H, J=12 Hz), 2.56-2.61 (m, 1H), 3.11-3.18 (m, 1H), 3.36-3.40 (m, 1H), 3.48 (t, 1H, J=12 Hz), 3.88-3.94 (m, 4H), 4.30-4.35 (m, 1H), 4.53 (d, 1H, J=12 Hz), 7.14-7.23 (m, 2H), 7.26-7.30 (m, 1H), 7.88 (s, 1H). LC-MS: 399.04 (M+1).
Intermediate 2
tert-Butyl[(2R,3S)-5-oxo-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate Step A: 1-(2,5-Difluorophenyl)-2-nitroethanol
To sodium hydroxide (1N, 3L) and methanol (1500 mL) at 5° C. was added a solution of 2,5-difluorobenzaldehyde (350 g, 2.46 mol) and nitromethane (157 mL, 2.9 mol) in methanol (350 mL) dropwise over a period of 1 h. The reaction mixture was then neutralized with glacial acetic acid (165 mL). Diethyl ether (1500 mL) was added and the layers separated. The organic layer was washed successively with saturated aqueous sodium carbonate solution (1000 mL), and saturated aqueous brine (1000 mL). The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated to afford 1-(2,5-difluorophenyl)-2-nitroethanol that was used without further purification in Step B.
Step B: 2-Nitro-1-(2,5-difluorophenyl)ethanone
A solution of Dess-Martin periodinane (125 g) in dichloromethane (600 mL) was added to a solution of the nitroalcohol made in Step A (46.3 g) at 10° C. over a period of 30 min. Stirring was continued for 2 h, and the reaction mixture was then poured onto a mixture of sodium bicarbonate (300 g) and sodium thiosulfate (333 g) in water (3 L). The desired product was extracted with methyl t-butyl ether (MTBE) (2 L). The aqueous layer was neutralized with HCl (2N, 1.5 L) and extracted with MTBE (3 L). The combined organic layers were dried over anhydrous magnesium sulfate, filtered, evaporated and the residue was purified by chromatography (silica gel, eluting with dichloromethane) to yield the desired nitroketone.
Step C: 3-Iodo-2-(iodomethyl)prop-1-ene
A mixture of 3-chloro-2-(chloromethyl)prop-1-ene (1.0 g, 8 mmol) and sodium iodide (6.6 g, 44 mmol) in acetone (60 mL) was stirred at room temperature for 20 h, evaporated under reduced pressure and partitioned between dichloromethane (150 mL) and water (50 mL). The organic layer was dried over sodium sulfate, filtered and evaporated to yield 3-iodo-2-(iodomethyl)prop-1-ene as a reddish oil.
Step D: 3-Methylene-5-nitro-6-(2,5-difluorophenyl)-3,4-dihydro-2H-pyran
N,N-diisopropylethylamine (184 mL) was added to a solution of 2-nitro-1-(2,5-difluorophenyl)ethanone (92.7 g, 461 mmol) in N,N-dimethylformamide (1000 mL) and 3-iodo-2-(iodomethyl)prop-1-ene (156 g, 507 mmol). The mixture was heated at 60° C. for 2 h, evaporated and purified by chromatography (silica gel, gradient 0-30% dichloromethane in hexane) to yield 3-methylene-5-nitro-6-(2,5-difluorophenyl)-3,4-dihydro-2H-pyran.
Step E: (2R,3S)-5-Methylene-3-nitro-2-(2,5-difluorophenyl)tetrahydro-2H-pyran
This compound was made by following the same method described in Intermediate 1, Step D by using 3-methylene-5-nitro-6-(2,5-trifluorophenyl)-3,4-dihydro-2H-pyran.
Step F: (2R,3S)-5-Methylene-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-amine
This compound was made by following the same method described in Intermediate 1, Step E by using (2R,3S)-5-Methylene-3-nitro-2-(2,5-difluorophenyl)tetrahydro-2H-pyran.
Step G: tert-Butyl[(2R,3S)-5-methylene-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate
This compound was made by following the same method described in Intermediate 1, Step F by using (2R,35)-5-methylene-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-amine.
Step H: tert-Butyl[(2R,3S)-5-hydroxy-5-(hydroxymethyl)-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate
This compound was made by following the same method described in Intermediate 1, Step G by using tert-butyl[(2R,35)-5-methylene-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate.
Step I: tert-Butyl[(2R,3S)-5-oxo-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate
To a solution of tert-butyl[(2R,3S)-5-hydroxy-5-(hydroxymethyl)-2-(2,5-trifluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate (10.5 g) in methanol (100 mL) at 0° C. was added pyridine (7.8 mL) and lead tetraacetate (21.7 g). The reaction mixture was stirred for 20 min. Aqueous work-up with ethyl acetate gave crude product which was purified by chromatography (silica, 0-50% ethyl acetate/heptane) to yield tert-butyl[(2R,35)-5-oxo-2-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl]carbamate as white solid.
Intermediate 3
Step A: tert-Butyl (3Z)-3-[(dimethylamino)methylene]-4-oxopyrrolidine-1-carboxylate
A solution of tert-butyl 3-oxopyrrolidine-1-carboxylate (40 g, 216 mmol) was treated with DMF-DMA (267 g, 2241 mmol) and heated at 105° C. for 40 min. The solution was cooled and evaporated under reduced pressure and the resulting orange solid was treated with hexane (200 mL) and cooled in a refrigerator for 3 days. The resulting brownish-yellow solid obtained as such was collected by filtration, dried and used in the next step without further purification.
Step B: 1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole
A solution of hydrazine (3 mL) and tert-butyl (3Z)-3-[(dimethylamino)methylene]-4-oxopyrrolidine-1-carboxylate (19.22 g) in ethanol (40 mL) was heated at 85° C. in a sealed tube for 4 h. Solvent was removed under reduced pressure, and the residue was triturated with dichloromethane (160 mL) and ethyl acetate (15 mL). The resulting solid was filtered. The filtrate was concentrated and the resulting solid was triturated again and filtered. The combined solids were treated with 4N hydrochloric acid (250 mL) in methanol and stirred for 6 h. The reaction mixture was concentrated and dried. The resulting solid was treated again for 6 h with 4N hydrochloric acid (250 mL) in methanol. After concentration and drying, the resulting hydrochloride salt was treated with ammonia in methanol (2N, 300 mL) and ammonium hydroxide solution in water (28%, 30 mL) and concentrated to dryness. The solid obtained was treated with methanol (70 mL) and water (5 mL) and purified in three batches on Biotage Horizon® system (silica, gradient 5-17% methanol containing 10% concentrated ammonium hydroxide in ethyl acetate) to yield 1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole. 1H NMR (500 MHz, CD3OD): δ 4.04 (d, 4H); 7.39 (s, 1H).
Intermediate 5
2-(Methylsulfonyl)-2,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole Step A: tert-Butyl 1-(methylsulfonyl)]-4,6-dihydropyrrolo[3,4-c]pyrazole-5(1H)-carboxylate (A) and tent-butyl 2-(methylsulfonyl)]-2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate (B)
A suspension of N-Boc-pyrazolopyrrolidine (Intermediate 3, Step B) (27.16 g, 130 mmol) in anhydrous acetonitrile (1.0 L) was charged in a 2.0 L three-neck flask fitted with a thermometer and an addition funnel and then treated with sodium hydride (60% dispersion in oil, 6.23 g, 156 mmol) while under nitrogen atmosphere in one portion. The reaction mixture was stirred at room temperature for 2 h. The resulting white suspension was then cooled in an ice bath and methanesulfonyl chloride (25.2 mL, 324 mmol) was slowly added via addition funnel The ice bath was then removed and the mixture was stirred 1 h at room temperature. The reaction mixture was quenched with water (500 mL) and the layers were separated. The aqueous layer was then extracted with 2×500 mL of dichloromethane. The combined organic layers were dried over sodium sulfate and concentrated under reduced pressure to give a mixture of products A and B as colorless syrups. NMR in CD3OD indicated a 1:1 mixture of two products, in which the proton on the pyrazole ring in product A appeared at 7.70 ppm while the proton in product B appeared at 7.95 pm. LC-MS: 288.08 (M+1).
Step B: 2-(Methylsulfonyl)-2,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole
Trifluoroacetic acid (200 mL) was added slowly to a solution containing intermediates A and B prepared in the previous step (48.4 g, 168 mmol) in dichloromethane (400 mL) at 0° C. After addition, the cooling bath was removed and the reaction was allowed to stir at room temperature for 2 h. Solvent was removed under reduced pressure and the resulting trifluoroacetate salt was then neutralized with 500 mL of 25% methanol and 2.5% ammonium hydroxide in dichloromethane. After removal of solvent, the desired Intermediate 5 was obtained after chromatography on a Biotage™ column (2×340 g) eluting with 2.5-12.5% methanol and 0.25-1.25% ammonium hydroxide in dichloromethane. LC-MS: 109.85 (M+1).
………………………………..
below patent caution…………….similar not same….examples below will help you in synthesis similarities
Step 1 2 Step 2
Example 1
Synthesis of 3: (Step 1 & 2)
Dimethyldisulfide 1 (5 g, 53 mmol) and acetic acid (6 mL, 106 mmol) were mixed under nitrogen atmosphere and cooled to – 20 °C. Sulfuryl chloride (13 mL, 159 mmol) was added dropwise with stirring. The mixture was then stirred for 1 hour at -20 °C and afterwards allowed to come to room temperature and continued for another two hours. Acetyl chloride was distilled off from the reaction mixture. Crude methanesulfinyl chloride 2 obtained was used in the next step without further purification.
To a solution of chloramine T (14.95 g, 53 mmol) in dry toluene (220 mL) was added a solution of methanesulfinyl chloride 2 (5.2 g, 53 mmol) in dry toluene (10 mL) at 0 °C. The resulting suspension was heated at 80 °C for 2 hours with stirring. After cooling, the solid was filtered off and washed with dry toluene (100 mL). The filtrate was evaporated in vacuo and the crude mixture was purified through silica gel chromatography to obtain 3 as off white solid. XH NMR (300 MHz, CDC13): δ 7.85 – 7.91 (m, J= 8.42 Hz, 2H), 7.31 – 7.38 (m, J= 8.23 Hz, 2H), 3.78 (s, 3H), 2.45 (s, 3H).
Synthesis of 4: (Step 3)
To a solution of Ml (1.0 g, 2.2 mmol) in THF (10 mL) and DMF (10 mL) under nitrogen atmosphere at 0 °C was added Et3N (0.92 mL, 6.6 mmol) followed by B0C2O (0.48 g, 2.2 mmol). The reaction mixture was allowed to come to room temperature and continued the stirring for over night. The reaction mixture was diluted with water (100 mL) and extracted with CH2CI2 (3 x 100 mL). Combined organics were dried over Na2S04, filtered, concentrated under vacuum and purified by silica gel chromatography afforded 4 as a off white solid.
XH NMR (400 MHz, CDC13): δ 7.27 – 7.35 (m, 1H), 4.44 – 4.54 (m, 4H), 1.52 (s, 9H).
Synthesis of 5: (Step 4)
To a suspension of NaH (0.30 g, 7.5 mmol) in dry THF (5 mL) under nitrogen atmosphere at 0 °C was added a solution of 4 (0.78 g, 3.7 mmol) in dry THF (30 mL). The reaction mixture was allowed to come to room temperature and continued the stirring for 2 hours. Reaction mixture was again cooled to 0 °C. A solution of 3 (2.0 g, 7.4 mmol) in THF (25 mL) was added to the reaction mixture and continued the stirring for another 1 hour. The reaction mixture was quenched with water (100 mL) and extracted with EtOAc (3 x 200 mL). Combined organics were dried over Na2S04, filtered, concentrated under vacuum and purified by silica gel chromatography afforded 5 as an off-white solid.
XH NMR (400 MHz, CDC13): δ 7.84 – 7.88 (m, 1H), 7.78 (t, J= 8.27 Hz, 2H), 7.23 – 7.30 (m, 2H), 4.39 – 4.49 (m, 4H), 3.53 (d, J= 2.40 Hz, 3H), 2.42 (s, 3H), 1.53 (s, 9H).; Molecular Formula: Ci8H24N405S2; LCMS purity: 98.18%; Expected: 440.1 ; Observed: 341.0 (M-99).
Synthesis of 6: (Step 5)
To a solution of 5 (0.47 g, 1.06 mmol) in dry CH2CI2 (1 1 mL) under nitrogen atmosphere at 0 °C was added TFA (3 mL). The reaction mixture was allowed to come to room temperature and continued the stirring for 2 hours. Solvent was removed under vacuum and solid mass was washed with Et20 (3 x 10 mL) to get amine TFA salt as white solid.
XH NMR (300 MHz, CD3OD): δ 7.78 (s, 1H), 7.63 – 7.70 (m, J= 8.11 Hz, 2H), 7.26 – 7.35 (m, J = 8.33 Hz, 2H), 3.93 (s, 2H), 3.86 (s, 2H), 3.34 (s, 3H), 2.42 (s, 3H).
The amine TFA salt was dissolved in minimum volume of MeOH:CHCi3 (1 : 1) and passed through a column [Orochem 5 g, 10 ml, Amino (N¾)] using MeOH as eluent.
Organics were concentrated under vacuum to get free 6.
Synthesis of 7: (Step 6) To a stirred solution of 6 (0.34 g, 0.95 mmol) and M2 (0.26 g, 0.79 mmol) in DMAc (6.78 mL) under nitrogen atmosphere for 10 minutes was added AcOH (0.067 mL, 1.19 mmol). The reaction mixture was stirred for further 5 minutes and cooled to 0 °C. NaBH(OAc)3 (0.20 g, 0.95 mmol) was added to the reaction mixture and allowed to stirrer at room temperature for overnight. NH4OH (2 mL) was added to the reaction mixture and heated at 50 °C for 1 hour followed by water (3.39 mL) and again heated at 50 °C for another hour. Reaction mixture was cooled to room temperature and filtered. The solid residue was washed with water (4 x 100 mL) and the crude residue was purified by silica gel chromatography to afford 7.
XH NMR (300 MHz, CDC13): δ 7.80 (d, J= 6.95 Hz, 3H), 7.25 – 7.29 (m, 2H), 7.22 (br. s., 1H), 6.92 – 7.02 (m, 2H), 4.52 (d, J= 9.33 Hz, 1H), 4.24 – 4.40 (m, 2H), 3.85 (br. s., 5H), 3.48 (s, 3H), 3.39 – 3.47 (m, 1H), 3.07 (br. s., 1H), 2.52 (d, J= 10.25 Hz, 1H), 2.44 (s, 3H), 1.61 (br. s., 1H), 1.28 (s, 9H).; Molecular Formula: C29H35F2N506S2; LCMS purity: 99.08%; Expected: 651.2; Observed: 652.0 (M+l). Synthesis of Example 1: (Step 7)
To a solution of 7 (20 mg, 0.03 mmol) in dry CH2CI2 (2 mL) under nitrogen atmosphere at 0 °C was added TFA (0.5 mL). The reaction mixture was allowed to come to room temperature and continued the stirring for 2 hours. Solvent was removed under vacuum and solid mass was washed with Et20 to get amine di-TFA salt Example 1 as white solid. Unless otherwise noted the IC50 values were determined using the assay discussed earlier.
XH NMR (400 MHz, CD3OD): δ 8.05 (s, 1H), 7.73 (d, J= 8.03 Hz, 2H), 7.36 (d, J= 8.28 Hz, 2H), 7.29 – 7.34 (m, 1H), 7.20 – 7.27 (m, 2H), 4.71 (d, J= 10.04 Hz, 1H), 4.40 – 4.53 (m, 5H), 3.72 – 3.82 (m, 2H), 3.68 (s, 3H), 3.59 – 3.65 (m, 1H), 2.77 – 2.85 (m, 1H), 2.44 (s, 3H), 2.00 – 2.14 (m, 1H).; Molecular Formula: C24H27F2 504S2; HPLC purity: 99.74%; LCMS Expected: 551.2; Observed: 552.2 (M+l).
SCHEME 2
Example 2: Synthesis of Compound 1 & 2 (Step 1):
To a suspension of M2 (0.95 g, 2.8 mmol) in water (8.67 mL) was added sodium metabisulfite (0.55 g, 2.8 mmol) and stirred a room temperature for lhour. A solution of M3* (0.52 g, 2.8 mmol) in ethanol (8.67 mL) was added to the above reaction mixture and continued the stirring for further 4 hours. Neat aCN (0.14 g, 2.8 mmol) was added to the above reaction mixture in one portion and heated the reaction mixture at 50 °C for 2 days. Reaction mixture was concentrated under vacuum to remove most of the ethanol. The crude mixture was extracted with CHCI3 (50 x 3 mL). The combined organic layer was washed with water, dried over a2S04, filtered, concentrated and purified by flash chromatography to obtain 1 and 2 as solids.
Compound 1: ‘H NMR (300 MHz, CDC13): δ 7.77 (s, 1H), 7.26 – 7.35 (m, 1H), 7.00 (t, J= 5.76 Hz, 2H), 4.57 (t, J= 9.88 Hz, 2H), 4.32 – 4.39 (m, 1H), 3.85 – 4.09 (m, 5H), 3.60 (d, J= 11.34 Hz, 1H), 3.34 (s, 3H), 2.63 – 2.74 (m, 1H), 2.02 – 2.15 (m, 1H), 1.31 (s, 9H).
Compound 2: XH NMR (300 MHz, CDC13): δ 7.28 – 7.36 (m, 2H), 7.00 (t, J= 5.85 Hz, 2H), 4.55 (d, J= 8.97 Hz, 2H), 4.37 (dd, J= 2.65, 11.25 Hz, 1H), 3.88 – 4.07 (m, 5H), 3.60 (d, J = 1 1.34 Hz, 1H), 2.71 (td, J= 3.45, 12.49 Hz, 1H), 1.97 – 2.12 (m, 1H), 1.31 (s, 9H).; Molecular Formula: C22H25F2 503; LCMS purity: 94.48%; Expected: 445.2; Observed: 446.0 (M+l). (*Preparation of M3: M3.PI1SO3H (1.0 g, 2.8 mmol) was dissolved in minimum volume of MeOH:CHCl3 (1 : 1) and passed through a column [Orochem 5 g, 10 ml, Amino (NH2)] using MeOH as eluent. Organics were concentrated under vacuum to get free M3, which was used directly without further purification.) Synthesis of compound 3 (Step 2):
To a solution of compound 2 (0.40 g, 0.89 mmol) in THF (5 mL) under 2 atmosphere at -78 °C was added a solution of MeMgBr (0.89 mL, 2.6 mmol, 3M in Et20). The reaction mixture was allowed to attain room temperature over 1 hour. TLC shows complete conversion. The reaction mixture was again cooled to -10 °C and quenched with saturated aq. NH4CI solution (10 mL). The reaction mixture was extracted with CH2CI2 (50 x 3 mL).
Combined organics were dried over Na2S04, filtered, concentrated and purified by reversed phase chromatography to obtain 3 as di-TFA salt.
Molecular Formula: C22H28F2 4O3; LCMS purity: 88.82%; Expected: 434.2; Observed: 435.2 (M+l).
Synthesis of Example 2 (Step 3):
To a solution of compound 3 (35 mg, 0.053 mmol) in CH2CI2 (2 mL) was added TFA (0.5 mL) dropwise at 0 °C. Reaction mixture was allowed to attain room temperature over 2 hours time. TLC shows complete conversion. Reaction mixture was concentrated to dryness. The solid residue was washed with Et20 (10 x 3 mL) and dried under vacuum to obtain Example 2 as tri-TFA salt.
XH NMR (400 MHz, CD3OD): δ 7.60 (s, 1H), 7.37 (dd, J= 5.02, 8.03 Hz, 1H), 7.22 – 7.31 (m, 2H), 4.70 (d, J= 10.04 Hz, 1H), 4.48 – 4.61 (m, 4H), 4.17 (dd, J= 2.26, 11.29 Hz, 1H), 3.91 (d, J = 11.04 Hz, 1H), 3.73 – 3.83 (m, 1H), 2.54 – 2.62 (m, 1H), 2.22 (t, J= 12.05 Hz, 1H), 1.71 (s, 3H).; Molecular Formula: C17H20F2 4O; HPLC purity: 94.98%; Expected: 334.2; Observed: 335.2 (M+l).
SCHEME 3
Example 3
Synthesis of 1 & 2: (Step 1)
To a suspension of M2 (0.95 g, 2.8 mmol) in water (8.67 mL) was added sodium metabisulfite (0.55 g, 2.8 mmol) and stirred a room temperature for lhour. A solution of M3* (0.52 g, 2.8 mmol) in ethanol (8.67 mL) was added to the above reaction mixture and continued the stirring for further 4 hours. Neat aCN (0.14 g, 2.8 mmol) was added to the above reaction mixture in one portion and heated the reaction mixture at 50 °C for 2 days. Reaction mixture was concentrated under vacuum to remove most of the ethanol. The crude mixture was extracted with CHCI3 (50 x 3 mL). The combined organic layer was washed with water, dried over a2S04, filtered, concentrated and purified by flash chromatography to obtain 1 and 2 as solids.
Compound 1: ‘H NMR (300 MHz, CDC13): δ 7.77 (s, 1H), 7.35 – 7.26 (m, 1H), 7.00 (t, J= 5.76 Hz, 2H), 4.57 (t, J= 9.88 Hz, 2H), 4.39 – 4.32 (m, 1H), 4.09 – 3.85 (m, 5H), 3.60 (d, J= 1 1.34 Hz, 1H), 3.34 (s, 3H), 2.74 – 2.63 (m, 1H), 2.15 – 2.02 (m, 1H), 1.31 (s, 9H).
Compound 2: XH NMR (300 MHz, CDC13): δ 7.36 – 7.28 (m, 2H), 7.00 (t, J= 5.85 Hz, 2H), 4.55 (d, J= 8.97 Hz, 2H), 4.37 (dd, J= 2.65, 11.25 Hz, 1H), 4.07 – 3.88 (m, 5H), 3.60 (d, J= 1 1.34 Hz, 1H), 2.71 (td, J= 3.45, 12.49 Hz, 1H), 2.12 – 1.97 (m, 1H), 1.31 (s, 9H).; Molecular Formula: C22H25F2 503; LCMS purity: 94.48%; Expected: 445.2; Observed: 446.0 (M+l).
(*Preparation of M3: M3.PI1SO3H (1.0 g, 2.8 mmol) was dissolved in minimum volume of MeOH:CHCl3 (1 : 1) and passed through a column [Orochem 5 g, 10 ml, Amino (NH2)] using MeOH as eluent. Organics were concentrated under vacuum to get free M3, which was used directly without further purification.) Synthesis of compound 3 (Step 2):
To a solution of 2 (0.40 g, 0.89 mmol) in THF (5 niL) under 2 atmosphere at -78 °C was added a solution of MeMgBr (0.89 mL, 2.6 mmol, 3M in Et20). The reaction mixture was allowed to attain room temperature over 1 hour. TLC shows complete conversion. The reaction mixture was again cooled to -10 °C and quenched with saturated aq. NH4CI solution (10 mL). The reaction mixture was extracted with CH2CI2 (50 x 3 mL). Combined organics were dried over Na2S04, filtered, concentrated and purified by reversed phase chromatography to obtain 3 (0.05 g, 8.4%) as di-TFA salt.
Molecular Formula: C22H28F2 4O3; LCMS purity: 88.82%; Expected: 434.2; Observed: 435.2 (M+l).
Synthesis of compound 4 (Step 3):
To a suspension of NaH (22 mg, 0.55 mmol) in dry THF (0.1 mL) under nitrogen atmosphere at 0 °C was added a solution of 3 (120 mg, 0.27 mmol) in dry THF (4.8 mL). The reaction mixture was allowed to come to room temperature and continued the stirring for 2 hours. Reaction mixture was again cooled to 0 °C. Methanesulfonyl chloride (0.42 mL, 0.55 mmol) was added to the reaction mixture and continued the stirring for another 1 hour. The reaction mixture was quenched with water and extracted with EtOAc (3 x 50 mL). Combined organics were dried over Na2S04, filtered, concentrated under vacuum and purified by silica gel chromatography afforded 4 as off white solid.
Molecular Formula: C23H30F2N4O5S; LCMS purity: 95.64%; Expected: 512.2; Observed: 513.2 (M+l). Synthesis of Example 3: (Step 4)
To a stirred solution of compound 4 (9.0 mg, 0.017 mmol) in CH2CI2 (2.0 mL) was added TFA (0.2 mL) dropwise at 0 °C. Reaction mixture was allowed to attain room temperature over 2 hours time. TLC shows complete conversion. Reaction mixture was concentrated to dryness. The solid residue was washed with Et20 (2 x 10 mL) and dried under vacuum. The solids were once again washed with a mixture of CH2CI2 (0.1 mL) and Et20 (5.0 mL) to obtain Example 3 (8.0 mg, 72.7%) as di-TFA salt. The IC50 value of Example 3 is 4nM. ¾ NMR (400MHz ,CD3OD): δ 7.96 (s, 1 H), 7.41 – 7.31 (m, 1 H), 7.30 – 7.19 (m, 2 H), 4.68 – 4.60 (m, 1 H), 4.22 – 4.07 (m, 4 H), 4.01 (d, J= 11.0 Hz, 1 H), 3.77 (d, J= 11.0 Hz, 1 H), 3.74 – 3.63 (m, 1 H), 3.39 (s, 3 H), 2.43 (d, J= 10.8 Hz, 1 H), 2.04 (t, J= 11.9 Hz, 1 H), 1.51 (s, 3 H).; Molecular Formula: C18H22F2 4O3S; HPLC purity: 95.01%; LCMS mass Expected: 412.2;
Observed: 413.0 (M+l).
Tesfaye Biftu et al, Omarigliptin (MK-3102): A Novel Long-Acting DPP-4 Inhibitor for Once-Weekly Treatment of Type 2 Diabetes;Journal of Medicinal Chemistry, Articles ASAP, March 24, 2014,DOI: 10.1021/jm401992e
Zacuto, Michael J. et al, Process for preparing chiral dipeptidyl peptidase-IV inhibitors;PCT Int. Appl., WO2013003250
Biftu, Tesfaye et al, Novel tetrahydropyran analogs as dipeptidyl peptidase IV inhibitors: Profile of clinical candidate (2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine, Bioorganic & Medicinal Chemistry Letters, 23(19), 5361-5366; 2013
Biftu, Tesfaye et al, Preparation of aminotetrahydropyrans as dipeptidyl peptidase IV inhibitors for the treatment or prevention of diabetes,PCT Int. Appl., WO2011028455
Biftu, Tesfaye et al, Preparation of aminotetrahydropyrans as dipeptidyl peptidase IV inhibitors for treatment or prevention of diabetes,U.S. Pat. Appl. Publ., US20100120863
Biftu, Tesfaye et al, Preparation of aminotetrahydropyrans as dipeptidyl peptidase IV inhibitors for treatment or prevention of diabetes,U.S. Pat. Appl. Publ., US20100120863
Xu, Feng et al, Process for preparation of chiral trans-2,3-disubstituted 5-oxotetrahydropyrans from ethyl N-(diphenylmethylene)glycinate and propargyl besylate, U.S. Pat. Appl. Publ., US20090187028
Ru(p-cymene)-N-sulfonyl-l,2-diphenylethylenediamine (DPEN) catalyst
R. Noyori, et al., J. Org. Chem., 66: 7931-7944 (2001)
B. Mohar, et al., Chem. Commun., 2572-2573 (2001)
R. Noyori, et al., J. Org. Chem., 66: 7931-7944 (2001)
B. Mohar, et al., Chem. Commun., 2572-2573 (2001)
The rhodium-catalyzed cycloisomerization
B. Trost etal., J.Amer. Chem.Soc., 125:7482-7483 (2003).
B. Trost etal., J.Amer. Chem.Soc., 125:7482-7483 (2003).
The ruthenium-catalyzed cycloisomerization
B. Trost, et al., J. Amer. Chem. Soc., 124: 2528-2533 (2002)
B. Trost, et al., J. Amer. Chem. Soc., 124: 2528-2533 (2002)
Gantz, I.; Chen, M.; Mirza, A.; Suryawanshi, S.; Davies, M. J.; Goldstein, B. J. Effect of MK-3102, a novel once-weekly DPP-4 inhibitor, over 12 weeks in patients with type 2 diabetes mellitus. Presented at the 48th Annual Meeting of the European Association for the Study of Diabetes (EASD), Berlin, Germany, October 2012; Abstract 101 (Clinical Research, Metabolism, Merck Research Laboratories).
3
RETAGLIPTIN
| Jiangsu Hengrui Medicine Co., Ltd |
- EP2436684A1
- Example 1. Preparation of hydrochloride of compound A (SP2086-HCL)
- (R)-7-[3-t-butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester (SM2086-15) (1.35kg, 2.40mol), HCL-ethyl acetate (greater than 2M) (12.3kg) were added into a 100L reaction kettle and stirred to dissolved. The mixture was reacted for more than 2 hours at normal temperature. Detected with TLC to reaction completely before evaporated and pumped to dryness with oil pump to give 1.15∼1.20kg of white to light yellow solid product with [α]
D20
- -28.0∼-33.0° (C=1, methanol), yield 96.0∼100%. The product was hydrochloride of (R)-7-[3-amino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7, 8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester (SP2086-HCL). (TLC detection: silica gel GF254plate; developing reagent: chloroform: methanol: ammonia= 40: 1: 0.1; raw material 15: Rf=0.80, product 1: Rf=0.50; ultraviolet visualization).
Example 2. Preparation of phosphate of compound A (SP2086-HPO4)
- SP2086-HCL(1.20kg, 2.40mol) was added into 100L reaction kettle, and dissolved in dichloromethane (15.2kg), then washed with saturated sodium bicarbonate solution (5.8kg). The aqueous layer was extracted once with dichloromethane ( 6.0 kg). The organic layers were combined and washed once with water (5kg), dried with anhydrous sodium sulphate. The mixture was filtrated and concentrated to dryness under reduced pressure at 40°C to give 1.12 kg of oil. The oil was stirred and dissolved with 30 times amount of isopropanol (26.0kg). A solution of 85% phosphoric acid (305.2g, 2.65mol) in isopropanol (1.22kg) was added immidiately after the oil completely dissolved. The solid was separated out, filtered after stirring for 2 hours and washed with cold isopropanol. The wet product was dried under reduced pressure at 40°C to give 1.16∼1.24kg of white to light yellow solid with a yield of 86.0∼92.0% (the wet product could be directly suspended in isopropanol without drying).
……………………………………… http://www.google.com/patents/EP2230241A1?cl=enExample 1(R)-7-[3-Amino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester hydrochloride
Step 1
- 2,2-Dimethyl-5-[2-(2,4,5-trifluoro-phenyl)-acetyl]-[1,3]dioxane-4,6-dione 2,2-Dimethyl-[1,3]dioxane-4,6-dione (5.69 g, 39.5 mmol) was dissolved in 400 mL of dichloromethane under stirring, followed by addition of (2,4,5-trifluoro-phenyl)-acetic acid 1a (7.15 g, 37.6 mmol) and 4-dimethylaminopyridine (7.35 g, 60.2 mmol) in an ice-water bath. Then a suspension of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (8.28 g, 43.2 mmol) in 250 mL of dichloromethane was added dropwise slowly. After stirring at room temperature for 36 hours, the reaction mixture was washed with the solution of 5% potassium bisulfate (250 mL×7) and saturated brine (250 mL×2), dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to obtain the title compound 2,2-dimethyl-5-[2-(2,4,5-trifluoro-phenyl)-acetyl]-[1,3]dioxane-4,6-dione 1b (11.4 g, yield 96%) as a white solid. MS m/z (ESI): 315.5 [M-1]
Step 23-Oxo-4-(2,4,5-trifluoro-phenyl)-butyric acid ethyl ester
- 2,2-Dimethyl-5-[2-(2,4,5-trifluoro-phenyl)-acetyl]-[1,3]dioxane-4,6-dione 1b (15.72 g, 49.6 mmol) was dissolved in 280 mL of ethanol under stirring, then the reaction mixture was heated to 70 °C in an oil bath overnight. After cooling, the mixture was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound 3-oxo-4-(2,4,5-trifluoro-phenyl)-butyric acid ethyl ester1c (12 g, yield 88%) as a yellow oil. MS m/z (ESI): 259 [M-1]
Step 33-Amino-4-(2,4,5-trifluoro-phenyl)-but-2-enoic acid ethyl ester
- 3-Oxo-4-(2,4,5-trifluoro-phenyl)-butyric acid ethyl ester 1c (24.6 g, 94.5 mmol) was dissolved in 240 mL of methanol, and ammonium acetate (36.4 g, 473 mmol) was added to the solution. The reaction mixture was heated to reflux for 3 hours and monitored by thin layer chromatography until the disappearance of the starting materials. The reaction mixture was concentrated under reduced pressure, then 100 mL of water was added to the residue. The mixture was extracted with ethyl acetate (200 mL×3), and the combined organic phase was washed with 200 mL of saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to obtain a light yellow solid. The resulting solid was dissolved in 50 mL of ethyl acetate at 80 °C, then 50 mL of n-hexane and seed-crystal were added to the solution. The mixture was cooled to room temperature, half an hour later, 100 mL of n-hexane was added. The mixture was stored in refrigerator overnight and then filtered under reduced pressure to obtain the title compound 3-amino-4-(2,4,5-trifluoro-phenyl)-but-2-enoic acid ethyl ester 1d(19.5 g, yield 80%) as a white solid. MS m/z (ESI): 260.1 [M+1]Step 43-tert-Butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyric acid ethyl ester
- 3-Amino-4-(2,4,5-trifluoro-phenyl)-but-2-enoic acid ethyl ester 1d(4.1 g, 15.8 mmol) was added into an autoclave, followed by addition of 70 mL of methanol, di-tert-butyl dicarbonate (3.8 g, 17.4 mmol), chloro(1, 5-cyclooctadiene)rhodium( I ) dimer (32 mg, 0.0632 mmol) and (R)-1-[(S)-2-(diphenyl phosphino)ferrocenyl]-ethyl-tert-butylphosphine (68 mg, 0.126 mmol). The reaction mixture was hydrogenated for 24 hours under 6.67 atmosphere at 30 °C. The mixture was filtered and the filtrate was concentrated under reduced pressure. Then 34 mL of methanol was added to the residue at 50 °C, followed by addition of 12 mL of water until all dissolved. After cooling to room temperature, the mixture was stored in the refrigeratory overnight and then filtered. The solid product was washed with the solvent mixture of methanol/water (v:v = 3:2), dried in vacuo to obtain the title compound 3-tert-butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyric acid ethyl ester 1e (4 g, yield 70%) as a light yellow solid. MS m/z (ESI): 362.4 [M+1]Step 5(R)-3-tert-Butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyric acid
- 3-tert-Butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyric acid ethyl ester 1e (10 g, 27.7 mmol) and sodium hydroxide (3.32 g, 83.1 mmol) were dissolved in the solvent mixture of 100 mL of methanol and 50 mL of water under stirring. The reaction mixture was reacted at 40-45 °C for 1-1.5 hours, then part of the solution was evaporated under reduced pressure. The residue was added with some water, then pH was adjusted to 2-3 with 1 N hydrochloric acid in an ice-water bath. The mixture was extracted with ethyl acetate (200 mLx3), and the combined organic phase was washed with 200 mL of saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, and then recrystallized from ethyl acetate/n-hexane to obtain the title compound (R)-3-tert-butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyric acid 1f(9.2 g) as a white solid, which was directly used in the next step. MS m/z (ESI): 332.3 [M-1] Reference: Tetrahedron Asymmetry, 2006, 17(2), 205-209
Step 6C-Pyrazin-2-yl-methylamine
- Pyrazine-2-carbonitrile 1g (10.5 g, 100 mmol) was dissolved in 150 mL of 1,4-dioxane under stirring, then Raney nickel (1.0 g) was added into a 250 mL autoclave. The reaction mixture was hydrogenated for 8 hours under 40 atmosphere at 60 °C, filtered and concentrated under reduced pressure to obtain the title compound C-pyrazin-2-yl-methylamine 1h (10.7 g, yield 98%) as a brown oil. MS m/z (ESI): 110 [M+1]
Step 72,2,2-Trifluoro-N-pyrazin-2-ylmethyl-acetamide
- C-Pyrazin-2-yl-methylamine 1h (10.9 g, 100 mmol) was added into a reaction flask, then 20 mL of trifluoroacetic anhydride was added dropwise slowly within an hour at 0 °C in an ice-water bath. The reaction mixture was reacted at room temperature for 2 hours and monitored by thin layer chromatography until the disappearance of the starting materials. Then it was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound 2,2,2-trifluoro-N-pyrazin-2-ylmethyl-acetamide 1i (21.0 g) as a brown oil. MS m/z (ESI): 206.1 [M+1]
Step 83-Trifluoromethyl-imidazo[1,5-a]pyrazine
- 2,2,2-Trifluoro-N-pyrazin-2-ylmethyl-acetamide 1i (21.0 g, 100 mmol) was added into a reaction flask at room temperature, followed by addition of 100 mL of phosphorus oxychloride. After stirring at room temperature for 30 minutes, phosphorous pentoxide (17.8 g, 125 mmol) was added to the solution. The reaction mixture was heated to reflux for 5 hours and monitored by thin layer chromatography until the disappearance of the starting materials. Phosphorus oxychloride was removed, and the reaction system was quenched with deionized water. The mixture was adjusted to pH 5-6 with 20% sodium hydroxide solution in an ice-water bath. The mixture was extracted with ethyl acetate (250 mL×4), and the combined organic phase was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound 3-trifluoromethyl-imidazo[1,5-a]pyrazine 1j (12.0 g, yield 65%) as a yellow solid. MS m/z (ESI): 188.0 [M+1] 1H NMR (400 MHz, CDCl3): δ 9.15 (s, 1H), 8.06 (d, 1H), 7.92 (s, 1H), 7.81 (d, 1H)
Step 93-Trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine
- 3-Trifluoromethyl-imidazo[1,5-a]pyrazine 1j (12.0 g, 64.2 mmol) was dissolved in 150 mL of anhydrous ethanol under stirring, then 10% Pd/C (500 mg) was added to the solution. The reaction mixture was stirred at room temperature under a hydrogen atmosphere overnight. The reaction solution was filtered through a pad of coarse silica gel and concentrated under reduced pressure to obtain the title compound 3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine 1k (12.2 g, yield 99%) as a brown solid. 1H NMR (400 MHz, CDCl3): δ 6.84 (s, 1H), 4.10 (m, 4H), 3.26 (m, 2H), 1.81 (s, 1H)
Step 10(R)-[3-Oxo-1-(2,4,5-trifluoro-benzyl)-3-(3-trifluoromethyl-5,6-dihydro-8H-imidazo [1,5-a]pyrazin-7-yl)-propyl]-carbamic acidtert-butyl ester
- Under a nitrogen atmosphere, 3-tert-butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyric acid 1k (8.6 g, 45 mmol) and 9.4 mL of triethylamine were dissolved in 300 mL of dichloromethane under stirring. After stirring at room temperature for 5 minutes, 3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine 1f (15.0 g, 45 mmol) and bis(2-oxo-3-oxazolidinyl)phosphonic chloride (17.1 g, 67.3 mmol) were added to the solution successively. The reaction mixture was reacted at room temperature for 2 hours and monitored by thin layer chromatography until the disappearance of the starting materials and then concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound (R)-[3-oxo-1-(2,4,5-trifluoro-benzyl)-3-(3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazin-7-yl)-propyl]-carbamic acid tert-butyl ester 1l (20.0 g, yield 88%) as a white solid. 1H NMR (400 MHz, CD3OD): δ 7.25 (m, 1H), 7.11 (m, 1H), 7.032 (s, 1H), 4.93 (m, 2H), 4.35 (m, 3H), 4.05 (m, 2H), 2.99 (m, 2H), 2.73 (m, 2H), 1.34 (s, 9H)
Step 11(R)-[3-(1-Bromo-3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazin-7-yl)-3-oxo-1-(2,4,5-trifluoro-benzyl)-propyl]-carbamic acidtert-butyl ester
- (R)-[3-Oxo-1-(2,4,5-trifluoro-benzyl)-3-(3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazin-7-yl)-propyl]-carbamic acid tert-butyl ester 11 (20.0 g, 39.6 mmol) was dissolved in 300 mL of anhydrous ethanol under stirring, and 1-bromo-2,5-pyrolidinedione (14.1 g, 79.2 mmol) was then added to the solution at room temperature. After stirring for an hour, potassium carbonate (10.9 g, 79.2 mmol) and di-tert-butyl dicarbonate (8.6 g, 39.6 mmol) were added to the mixture, and the mixture was stirred for an hour and monitored by thin layer chromatography until the disappearance of the starting materials. The reaction mixture was filtered through a pad of coarse silica gel to remove potassium carbonate, and then concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound (R)-[3-oxo-1-(2,4,5-trifluoro-benzyl)-3-(1-bromo-3-trifluoromethyl-5,6-dihydro-8H-i midazo [1,5-a]pyrazin-7-yl)-propyl]-carbamic acid tert-butyl ester1m (20.0 g, yield 86%) as a white solid. 1H NMR (400 MHz, CDCl3): δ 7.063 (m, 1H), 6.88 (m, 1H), 4.72 (s, 1H), 4.56 (s, 1H), 4.13 (m, 3H), 3.88 (m, 2H), 2.94 (m, 2H), 2.62 (m, 2H), 1.36 (s, 9H)
Step 12(R)-7-[3-tert-Butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester
- Octacarbonyldicobalt (4.02 g, 11.76 mmol), ethyl chloroacetate (0.71 g, 5.88 mmol), potassium carbonate (1.62 g, 11.76 mmol) and 50 mL of methanol were added into the reaction flask. After stirring for 5 minutes, (R)-[3-oxo-1-(2,4,5-trifluoro-benzyl)-3-(1-bromo-3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazin-7-yl)-propyl]-carbamic acidtert-butyl ester 1m (2.3 g, 3.92 mmol) was added. The reaction mixture was reacted at 60 °C in an oil bath, and the colour of the reaction mixture turned from puce to purple. 2 hours later, Electro-Spray Ionization (ESI) mass spectrometry showed the starting material disappeared. The reaction mixture was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain the title compound (R)-7-[3-tert-butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester 1n (1.1 g, yield 50%) as a white solid. MS m/z (ESI): 565.0 [M+1] Reference: Journal of Organometallic Chemistry, 1985, 285(1-3), 293-303
Step 13(R)-7-[3-Amino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester hydrochloride
- [0064](R)-7-[3-tert-Butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester 1n (0.12 g, 2.12 mmol) was added to a solution of 2.2 N hydrochloric acid in 5 mL of ethyl acetate. The reaction mixture was reacted at room temperature for 5 hours and monitored by thin layer chromatography until the disappearance of the starting materials. The reaction mixture was concentrated under reduced pressure to obtain the title compound (R)-7-[3-amino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester hydrochloride 1 (0.12 g, yield 94.3%) as a light yellow solid. MS m/z (ESI): 465.2 [M+1] 1H NMR (400 MHz, CD3OD): δ 7.101-7.08 (m, 1H), 6.906-6.864 (m, 1H), 5.343-4.995 (m, 2H), 4.221-4.093 (m, 5H), 3.954 (s, 3H), 2.978-2.937 (m, 2H), 2.71-2.643 (m, 2H), 2.061 (s, 2H)
| EP2230241A1 * | Nov 27, 2008 | Sep 22, 2010 | Jiangsu Hengrui Medicine Co., Ltd. | Tetrahydro-imidazoý1,5-a¨pyrazine derivatives, preparation methods and medical uses thereof |
| WO2003004498A1* | Jul 5, 2002 | Jan 16, 2003 | Merck & Co Inc | Beta-amino tetrahydroimidazo (1, 2-a) pyrazines and tetrahydrotrioazolo (4, 3-a) pyrazines as dipeptidyl peptidase inhibitors for the treatment or prevention of diabetes |
| WO2005003135A1* | Jun 18, 2004 | Jan 13, 2005 | Alex Minhua Chen | Phosphoric acid salt of a dipeptidyl peptidase-iv inhibitor |
4 CARMEGLIPTIN
(2S,3S,11βS)-1-(2-Amino-9,10-dimethoxy-1,3,4,6,7,11β-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-(4S)-fluoromethyl-pyrrolidin-2-one Dihydrochloride
(2S,3S,11bS)-1-(2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4(S)-fluoromethyl-pyrrolidin-2-one
813452-14-1 (di-HCl)
916069-91-5 (mono-HCl)
916069-91-5 (mono-HCl)
Roche…….innovator
CARMEGLIPTIN
813452-18-5
(2S,3S,11βS)-1-(2-Amino-9,10-dimethoxy-1,3,4,6,7,11β-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-(4S)-fluoromethyl-pyrrolidin-2-one
(S)-1-((2S,3S,11bS)-2-amino-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-3-yl)-4-(fluoromethyl)pyrrolidin-2-one
| (S)-1-((2S,3S,11bS)-2-amino-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-3-yl)-4-(fluoromethyl)pyrrolidin-2-one | |
| (S)-1-((2S,3S,11bS)-2-amino-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-3-yl)-4-(fluoromethyl)pyrrolidin-2-one | |
| 分子式: | C20H28FN3O3 |
| 分子量: | 377 |
813452-18-5, Carmegliptin, R-1579;carmegliptin, Carmegliptin (USAN/INN), SureCN419289, UNII-9Z723VGH7J, CHEMBL591118, CHEBI:699093, Ro-4876904, D08631, R-1579, B1Q
Type 2 diabetes is a chronic, progressive metabolic disease, affecting about 4% of the world population. The main goal of the management of type 2 diabetes is to achieve glycemic control as close to the nondiabetic range as practicable, in order to reduce the risk of late-stage complications.However, the therapeutic effect provided by existing medications is often not sustainable, since the multi-organ defects responsible for the disease are only insufficiently addressed.
Dipeptidyl peptidase-IV (DPP-IV) inhibitors have emerged as a new therapeutic option to treat type 2 diabetes.
Their rapid rise in popularity is due to the favourable safety profile (no hypoglycemia, no weight gain, no gastrointestinal problems—typical side effects associated with established anti-diabetic agents). DPP-IV is a ubiquitous serine protease, the inhibition of which prevents the degradation of glucagon-like peptide 1 (GLP-1). The resulting higher levels of GLP-1 have a beneficial impact on major players involved in the pathogenesis of type 2 diabetes: β-cells, liver, α-cells, gut, and brain.
Long-term studies with DPP-IV inhibitors in patients are underway in order to confirm the safety and sustainability of these effects, and, in particular, their ability to prevent the progressive loss of β-cell function.
SYNTHESIS
aReagents and conditions: a) HCO2Me, Δ; b) POCl3, MeCN; c) HO2CCH2CO2Et, neat, 120 °C; d) ethyl acrylate, neat; e) t-BuOK, neat (5 steps); f) NH4OAc, MeOH; g) NaBH4, TFA, THF; h) Boc2O, CH2Cl2; i) KOH, aq THF; j) DPPA, Et3N, TMSCH2CH2OH, PhMe, 80 °C; k) Et4NF, MeCN; l) chiral HPLC; m) Et3N, CH2Cl2; n) NaH, DMF; o) HCl, dioxane; p) HCl, 2-PrOH.
Scheme 2.
Reagents and conditions: (a) NH4OAc, MeOH, rt, 95%; (b) NaBH4, TFA, THF, 0 °C; (c) Boc2O, CH2Cl2, 83% over 2 steps; (d) KOH, aq THF, rt; (e) DPPA, Et3N, 2-(trimethylsilyl)ethanol, toluene, 80 °C; (f) Et4NF, CH3CN, 50 °C, 56% over 3 steps; (g) Et3N, CH2Cl2, (h) NaH, cat. NaI, DMF; (i) HCl, 1,4-dioxane.
Carmegliptin (2.70) is an anti-diabetes drug which is currently in late stage clinical trials. It represents a further structural advancement from the other existing marketed drugs in this class, sitagliptin (2.71, Januvia) and vildagliptin (2.72, Zomelis, Figure 7). These compounds are all members of the dipeptidyl peptidase 4 class (DPP-4), a transmembrane protein that is responsible for the degradation of incretins; hormones which up-regulate the concentration of insulin excreted in a cell. As DPP-4 specifically cleaves at proline residues, it is unsurprising that the members of this drug class exhibit an embedded pyrrolidine ring (or mimic) and additional decoration (a nitrile or fluorinated alkyl substituent is present in order to reach into a local lipophilic pocket). One specific structural liability of the 2-cyano-N-acylpyrrolidinyl motif (2.73) is its inherent susceptibility towards diketopiperazine formation (2.74, Scheme 29) [80], however, one way to inhibit this transformation is to position a bulky substituent on the secondary amine nucleophile as is the case in vildagliptine (2.72).
Figure 7: Structures of DPP-4 inhibitors of the gliptin-type.
Scheme 29: Formation of inactive diketopiperazines from cis-rotameric precursors.
A single crystal X-ray structure of carmegliptin bound in the human DPP-4 active site has been published indicating how the fluoromethylpyrrolidone moiety extends into an adjacent lipophilic pocket [81]. Additional binding is provided by π–π interaction between the aromatic substructure and an adjacent phenylalanine residue as well as through several H-bonds facilitated by the adjacent polar substituents (Figure 8).
Figure 8: Co-crystal structure of carmegliptin bound in the human DPP-4 active site (PDB 3kwf).
The reported synthesis of carmegliptin enlists a Bischler-Napieralski reaction utilising the primary amine 2.76 and methyl formate to yield the initial dihydroquinoline 2.77 as its HCl salt (Scheme 30) [82]. This compound was next treated with 3-oxoglutaric acid mono ethyl ester (2.78) in the presence of sodium acetate. Decarboxylation then yields the resulting aminoester 2.79 which was progressed through an intramolecular Mannich-type transformation using aqueous formaldehyde to allow isolation of enaminoester 2.80 after treatment of the intermediate with ammonium acetate in methanol.
The next step involves a very efficient crystallisation-induced dynamic resolution of the racemic material using the non-natural (S,S)-dibenzoyl-D-tartaric acid ((+)-DBTA). It is described that the desired (S)-enantiomer of compound 2.81 can be isolated in greater than 99% ee and 93% overall yield. This approach is certainly superior to the original separation of the two enantiomers (at the stage of the final product) by preparative chiral HPLC that was used in the discovery route (albeit it should be noted that both enantiomers were required for physiological profiling at the discovery stage).
Next, a 1,2-syndiastereoselective reduction of enaminoester 2.81 occurs with high diastereocontrol imposed by the convexed presentation of the substrate for the formal conjugate addition and subsequent protonation steps. This is followed by Boc-protection and interconversion of the ethyl ester to its amide derivative 2.82 in 80% overall yield for this telescoped process. The primary amide in2.82 was then oxidised via a modern variant of the classical Hoffmann rearrangement using phenyliodine diacetate (PIDA).
Following extensive investigation it was found that slowly adding this reagent in a mixture of acetonitrile/water to a suspension of amide2.82 and KOH gave clean conversion to the amine product in high yield. This new procedure was also readily scalable offering a cleaner, safer and more reliable transformation when compared to other related rearrangement reactions. During a further telescoped procedure amine 2.83 was treated with lactone 2.84 to regenerate the corresponding lactam after mesylate formation. Finally, removal of the Boc-group with aqueous hydrochloric acid furnished carmegliptin as its HCl salt.
Scheme 30: Improved route to carmegliptin.
- Peters, J.-U. Curr. Top. Med. Chem. 2007, 7, 579–595……………..80
- Mattei, P.; Boehringer, M.; Di Gorgio, P.; Fischer, H.; Hennig, M.; Huwyler, J.; Koçer, B.; Kuhn, B.; Loeffler, B. M.; MacDonald, A.; Narquizian, R.; Rauber, E.; Sebokova, E.; Sprecher, U.Bioorg. Med. Chem. Lett. 2010, 20, 1109–1113.doi:10.1016/j.bmcl.2009.12.024………..81
- Albrecht, S.; Adam, J.-M.; Bromberger, U.; Diodone, R.; Fettes, A.; Fischer, R.; Goeckel, V.; Hildbrand, S.; Moine, G.; Weber, M.Org. Process Res. Dev. 2011, 15, 503–514. doi:10.1021/op2000207……….82
………………………………………………………………………………………………………………..
Org. Process Res. Dev. 2011, 15, 503–514. doi:10.1021/op2000207
A short and high-yielding synthesis of carmegliptin (1) suitable for large-scale production is reported. The tricyclic core was assembled efficiently by a decarboxylative Mannich addition−Mannich cyclization sequence. Subsequent crystallization-induced dynamic resolution of enamine 7 using (S,S)-dibenzoyltartaric acid was followed by diastereoselective enamine reduction to give the fully functionalized tricyclic core with its three stereogenic centers. The C-3 nitrogen was introduced by Hofmann rearrangement of amide28, and the resulting amine 10was coupled with (S)-fluoromethyl lactone 31. Following cyclization to lactam 13 and amine deprotection, 1 was obtained in 27−31% overall yield with six isolated intermediates.
Preparation of (2S,3S,11βS)-1-(2-Amino-9,10-dimethoxy-1,3,4,6,7,11β-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-(4S)-fluoromethyl-pyrrolidin-2-one Dihydrochloride (1) CARMEGLIPTIN
A suspension of carbamate 13 (136 kg, 285 mol) in a mixture of H2O (112 kg) and acetone (122 kg) was treated at 50 °C within 60 min with 37% aq HCl (98.0 kg). After 90 min at 47−52 °C the solution was polish filtered through a 5 μm filter. The first reactor and the transfer lines were washed with a hot (47−52 °C) mixture of H2O (13.0 kg) and acetone (116 kg). The filtrate was cooled to 25 °C and treated at this temperature within 80 min with acetone (1600 kg) whereupon the product crystallized out. The resulting suspension was stirred for 1 h at 25 °C and subsequently centrifuged. The crystals were washed in two portions with acetone (391 kg) and dried at 50 °C and <30 mbar until constant weight to afford 122.4 kg (95%) of the title compound as colorless crystals with an assay (HPLC) of 98.8% (w/w).
1H NMR (400 MHz, D2O) δ 2.11−2.22 (m, 1H); 2.45 (dd, J = 17.6 Hz, 6.7 Hz; 1H); 2.76 (dd, J = 17.6 Hz, 9.55 Hz, 1H); 2.90−3.05 (m, 1H); 3.08−3.19 (m, 2H); 3.24−3.36 (m, 1H); 3.43 (dd, J = 9.8 Hz, 5.75 Hz, 1H); 3.49−3.58 (m, 1H); 3.70−3.84 (m, 4H); 3.87 (s, 3H); 3.88 (s, 3H); 4.12 (td, J = 11.6 Hz, 4.5 Hz, 1H); 4.45−4.55 (m, 1H); 4.56−4.68 (m, 3H); 6.91 (s, 1H), 6.95 (s, 1H).
IR (cm−1): 3237, 2925, 1682, 496, 478.
MS (ESI): m/z 378.3 ([M + H]+ (free amine)).
Anal. Calcd for C20H30Cl2FN3O3: C, 53.34; H, 6.71; N, 9.33; Cl, 15.74; F 4.22; O, 10.66. Found: C, 53.04; H, 6.43; N, 9.45; Cl, 15.66; F, 4.29; O, 11.09.
REF FOR ABOVE
Mattei, P.; Böhringer, M.; Di Giorgio, P.; Fischer, H.; Hennig, M.; Huwyler, J.; Kocer, B.; Kuhn, B.; Löffler, B. M.; MacDonald, A.; Narquizian, R.; Rauber, E.; Sebokova, E.; Sprecher, U. Bioorg. Med. Chem. Lett. 2010, 20, 1109
Böhringer, M.; Kuhn, B.; Lübbers, T.; Mattei, P.; Narquizian, R.; Wessel,H. P. (F. Hoffmann-La Roche AG). U.S. Pat. Appl. 2004/0259902, 2004.
…………………………………………………..
Discovery of carmegliptin: A potent and long-acting dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes
Bioorg Med Chem Lett 2010, 20(3): 1109
Bioorg Med Chem Lett 2010, 20(3): 1109
http://www.sciencedirect.com/science/article/pii/S0960894X09017296
Discovery of carmegliptin: A potent and long-acting dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes
- Pages 1109-1113
- Patrizio Mattei, Markus Boehringer, Patrick Di Giorgio, Holger Fischer, Michael Hennig, Joerg Huwyler, Buelent Koçer, Bernd Kuhn, Bernd M. Loeffler, Alexander MacDonald, Robert Narquizian, Etienne Rauber, Elena Sebokova, Urs Sprecher
Scheme 3.
Reagents and conditions: (a) preparative HPLC (Chiralpak® AD column), heptane/2-propanol 85:15, 37% (b) BH3.Me2S, THF, 0 °C; (c) (MeOCH2CH2)2NSF3, CH2Cl2, 67% (2 steps); (d), SOCl2, ZnCl2, 80 °C, 72 h, 61%; (e) Et3N, CH2Cl2; (f) NaH, DMF, 56% (2 steps); (g) HCl, 1,4-dioxane, 91%; (h) HCl, 2-propanol, 86%.
The synthesis of 8p is outlined ABOVE and required the enantiopure building blocks (S,S,S)-5 and 12. (S,S,S)-5 was obtained from the racemate by preparative chiral HPLC. Acid chloride 12 was prepared starting from (S)-paraconic acid (9). Reduction of 9 with borane–dimethyl sulfide complex afforded hydroxymethyl lactone 10. Since 10 is known to racemise rather readily, it was immediately treated with bis(2-methoxyethyl)aminosulfur trifluoride, thereby affording fluoromethyl lactone 11. This was converted to 12 by reaction with thionyl chloride in the presence of zinc chloride. The (S)-4-fluoromethyl-pyrrolidinone 8p was isolated as the dihydrochloride salt, a highly water soluble white crystalline solid, mp >275 °C.
…………………………………………………….
US 2013109859
The most preferred product is (2S,3S,11bS)-2-tert.-Butoxycarbonylamino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H pyrido[2,1-a]isoquinoline-3-carboxylic acid amide having the following structure:
It has been found that during the amidation of the ester epimerization takes place at position 3 and thus the 3R-epimer of the formula IVb is transformed to a larger extent in the 3S-epimer of formula V.
e) Preparation of (2S,3S,11bS)-1-(2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4(S)-fluoromethyl-pyrrolidin-2-one Dihydrochloride
A 2.5 L reactor equipped with a mechanical stirrer, a Pt-100 thermometer, a dropping funnel and a nitrogen inlet was charged with 619 g (1.30 mol) of (2S,3S,11bS)-3-((4S)-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester, 4.2 L isopropanol and 62 mL water and the suspension was heated to 40-45° C. In a second vessel, 1.98 L isopropanol was cooled to 0° C. and 461 mL (6.50 mol) acetyl chloride was added during 35 min, maintaining the temperature at 0-7° C. After completed addition, the mixture was allowed to reach ca. 15° C. and was then slowly added to the first vessel during 1.5 h. After completed addition the mixture was stirred for 18 h at 40-45° C., whereas crystallization started after 1 h. The white suspension was cooled to 20° C. during 2 h, stirred at that temperature for 1.5 h and filtered. The crystals were washed portionwise with 1.1 L isopropanol and dried for 72 h at 45° C./20 mbar, to give 583 g of the product as white crystals (100% yield; assay: 99.0%).
…………………………………………………….
US 2008071087
(2S,3S,11bS)-(3-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)]-carbamic acid tert-butyl ester (8)
Example 8
Transformation of (2S,3S,11bS)-(3-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl) ]-carbamic acid tert-butyl ester into (S)-1-((2S,3S,11bS)-2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl) -4-fluoromethyl-pyrrolidin-2-one.a)
Preparation of 4-fluoromethyl-5H-furan-2-oneA 6 L reactor equipped with a mechanical stirrer, a Pt-100 thermometer, a dropping funnel and a nitrogen inlet was charged with 500 g (4.38 mmol) 4-hydroxymethyl-5H-furan-2-one and 2.0 L dichloromethane. The solution was cooled to −10° C. and 1.12 kg (4.82 mol) bis-(2-methoxyethyl)aminosulfur trifluoride (Deoxo-Fluor) was added during 50 min, maintaining the temperature at −5 to −10° C. with a cooling bath. During the addition a yellowish emulsion formed, which dissolved to an orange-red solution after completed addition. This solution was stirred for 1.5 h at 15-20° C., then cooled to −10° C. A solution of 250 ml water in 1.00 L ethanol was added during 30 min, maintaining the temperature between −5 and −10° C., before the mixture was allowed to reach 15-20° C. It was then concentrated in a rotatory evaporator to a volume of ca. 1.6 L at 40° C./600-120 mbar. The residue was dissolved in 2.0 L dichloromethane and washed three times with 4.0 L 1N hydrochloric acid. The combined aqueous layers were extracted three times with 1.4 L dichloromethane. The combined organic layers were evaporated in a rotatory evaporator to give 681 g crude product as a dark brown liquid. This material was distilled over a Vigreux column at 0.1 mbar, the product fractions being collected between 71 and 75° C. (312 g). This material was re-distilled under the same conditions, the fractions being collected between 65 and 73° C., to give 299 g 4-fluoromethyl-5H-furan-2-one (58% yield; assay: 99%).MS: m/e 118 M+, 74,59,41.b) Preparation of (S)-4-fluoromethyl-dihydro-furan-2-oneA 2 L autoclave equipped with a mechanical stirrer was charged with a solution of 96.0 g 4-fluoromethyl-5H-furan-2-one (8.27×10−1 mol) in 284 mL methanol. The autoclave was sealed and pressurized several times with argon (7 bar) in order to remove any traces of oxygen. At ˜1 bar argon, a solution of 82.74 mg Ru(OAc)2((R)-3,5-tBu-MeOBlPHEP) (6.62×10−5 mol) (S/C 12500) in 100 mL methanol was added under stirring from a catalyst addition device previously charged in a glove box (O2 content <2 ppm) and pressurized with argon (7 bar). The argon atmosphere in the autoclave was replaced by hydrogen (5 bar). At this pressure, the reaction mixture was stirred (˜800 rpm) for 20 h at 30° C. and then removed from the autoclave and concentrated in vacuo. The residue was distilled to afford 91.8 g (94%) (S)-4-fluoromethyl-dihydro-furan-2-one. The chemical purity of the product was 99.7% by GC-area.c) Preparation of (2S,3S,11bS)-3-(3-Fluoromethyl-4-hydroxy-butyrylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl esterA 1.5 L reactor equipped with a mechanical stirrer, a Pt-100 thermometer, a dropping funnel and a nitrogen inlet was charged with 50 g (128 mmol) (2S,3S,11bS)-3-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)-carbamic acid tert-butyl ester, 500 mL toluene and 2.51 g (25.6 mmol) 2-hydroxypyridine. To this slightly brownish suspension, 22.7 g (192 mmol) of (S)-4-fluoromethyl-dihydro-furan-2-one was added dropwise at RT. No exothermy was observed during the addition. The dropping funnel was rinsed portionwise with totally 100 mL toluene. The suspension was heated to reflux, whereas it turned into a dear solution starting from 60° C., after 40 min under reflux a suspension formed again. After totally 23 h under reflux, the thick suspension was cooled to RT, diluted with 100 mL dichloromethane and stirred for 30 min at RT. After filtration, the filter cake was washed portionwise with totally 200 mL toluene, then portionwise with totally 100 mL dichloromethane. The filter cake was dried at 50° C./10 mbar for 20 h, to give 60.0 g product (94% yield; assay: 100%).
MS: m/e 496 (M+H)+, 437.
d) Preparation of (2S,3S,11bS)-3-((4S)-Fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl esterA 1.5 L reactor equipped with a mechanical stirrer, a Pt-100 thermometer, a dropping funnel, a cooling bath and a nitrogen inlet was charged with 28 g (56.5 mmol) of (2S,3S,11bS)-3-(3-fluoromethyl-4-hydroxy-butyrylamino) -9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester and 750 mL THF. The mixture was cooled to 0° C. and a solution of 6.17 mL (79 mmol) methanesulfonic acid in 42 mL THF was added during 10 min, maintaining the temperature at 0-5° C. At 0° C. a solution of 12.6 mL (90.2 mmol) triethylamine in 42 mL THF was added during 15 min. The resulting suspension was stirred for 80 min at 0-5° C., whereas it became gradually thicker. Then 141 mL (141 mmol) 1 M lithium-bis(trimethylsilyl)amide were added to the mixture during 15 min, whereas the suspension dissolved. The solution was allowed to reach RT during 60 min under stirring. 500 mL water was added without cooling, the mixture was extracted and the aqueous phase was subsequently extracted with 500 mL and 250 mL dichloromethane. The organic layers were each washed with 300 mL half saturated brine, combined and evaporated on a rotatory evaporator. The resulting foam was dissolved in 155 mL dichloromethane, filtered and again evaporated to give 30.5 g crude product as a slightly brownish foam. This material was dissolved in 122 mL methanol, resulting in a thick suspension, which dissolved on heating to reflux. After 20 min of reflux the solution was allowed to gradually cool to RT during 2 h, whereas crystallization started after 10 min. After 2 h the suspension was cooled to 0° C. for 1 h, followed by −25° C. for 1 h. The crystals were filtered off via a pre-cooled glass sinter funnel, washed portionwise with 78 mL TBME and dried for 18 h at 45° C./20 mbar, to give 21.0 g of the title product as white crystals (77% yield; assay: 99.5%).
MS: m/e 478 (M+H)+, 437, 422.
e) Preparation of (2S,3S,11bS)-1-(2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4(S)-fluoromethyl-pyrrolidin-2-one dihydrochlorideA 2.5 L reactor equipped with a mechanical stirrer, a Pt-100 thermometer, a dropping funnel and a nitrogen inlet was charged with 619 g (1.30 mol) of (2S,3S,11bS)-3-((4S)-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester, 4.2 L isopropanol and 62 mL water and the suspension was heated to 40-45° C. In a second vessel, 1.98 L isopropanol was cooled to 0° C. and 461 mL (6.50 mol) acetyl chloride was added during 35 min, maintaining the temperature at 0-7° C. After completed addition, the mixture was allowed to reach ca. 15° C. and was then slowly added to the first vessel during 1.5 h. After completed addition the mixture was stirred for 18 h at 40-45° C., whereas crystallization started after 1 h. The white suspension was cooled to 20° C. during 2 h, stirred at that temperature for 1.5 h and filtered. The crystals were washed portionwise with 1.1 L isopropanol and dried for 72 h at 45° C./20 mbar, to give 583 g of the product as white crystals (100% yield; assay: 99.0%).
These compounds are useful intermediates for the preparation of DPP-IV inhibitors as disclosed in PCT International Patent Appl. WO 2005/000848. More preferably, the invention relates to a process for the preparation of (2S,3S,11bS)-(3-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)]-carbamic acid tert-butyl ester.
XXXXXXX
According to still another embodiment (Scheme 2, below) the (S)-4-fluoromethyl-dihydro-furan-2-one (VII) is directly coupled with the amino-pyrido[2,1-a]isoquinoline derivative (VI) to form the hydroxymethyl derivative of the pyrido[2,1-a]isoquinoline (VIII), which is then subsequently cyclized to the fluoromethyl-pyrrolidin-2-one derivative (IX). The latter can be deprotected to yield the desired pyrido[2,1-a]isoquinoline derivative (I).
In a further preferable embodiment, the process for the preparation of (S)-1-((2S,3S,11bS)-2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one or of a pharmaceutically acceptable salt thereof comprises the subsequent steps:
- e) coupling of the (2S,3S,11bS)-3-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)-carbamic acid tert-butyl ester (amine of formula VI, wherein R2 and R3 are methoxy, R4 is hydrogen and Prot is Boc) with the (S)-4-fluoromethyl-dihydro-furan-2-one of formula
- f) cyclization of the obtained (2S,3S,11bS)-3-(3-fluoromethyl-4-hydroxy-butyrylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester in the presence of a base, and
- g) deprotecting the obtained (2S,3S,11bS)-3-((4S)-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester.
………………………………………………………….
PATENT
Example 23
RACEMIC
1-((RS,RS,RS)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one
a) 4-Fluoromethyl-dihydro-furan-2-one
A solution of 4-hydroxymethyl-dihydro-furan-2-one (Tetrahedron 1994, 50, 6839; 1.02 g, 8.78 mmol) and bis(2-methoxyethyl)aminosulfur trifluoride (3.88 g, 17.6 mmol) in chloroform (4.4 mL) was stirred at 40° C. for 1 h, then poured onto ice and partitioned between sat. aq. sodium hydrogencarbonate solution and dichloromethane. The organic layer was washed with brine, dried (MgSO4), and evaporated. Chromatography (SiO2, heptane-ethyl acetate gradient) afforded the title compound (576 mg, 56%). Colourless liquid, MS (EI) 118.9 (M+H)+.
b) 3-Chloromethyl-4-fluoro-butyryl chloride
A mixture of 4-fluoromethyl-dihydro-furan-2-one (871 mg, 7.37 mmol), thionyl chloride (4.39 g, 36.9 mmol), and zinc chloride (60 mg, 0.44 mmol) was stirred 72 h at 80° C., then excess thionyl chloride was removed by distillation. Kugelrohr distillation of the residue (85° C., 0.2 mbar) afforded the title compound (450 mg, 35%). Colourless liquid, 1H-NMR (300 MHz, CDCl3): 4.65–4.55 (m, 1H), 4.50–4.40 (m, 1H), 3.70–3.60 (m, 2H), 3.25–3.05 (m, 2H), 2.80–2.60 (m, 1H).
c) (RS,RS,RS)-[3-(3-Chloromethyl-4-fluoro-butyrylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester
The title compound was produced in accordance with the general method of Example 5c from (RS,RS,RS)-(3-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)-carbamic acid tert-butyl ester (Example 5b) and 3-chloromethyl-4-fluoro-butyryl chloride. White solid, MS (ISP) 514.5 (M+H)+.
d) (RS,RS,RS)-[3-(4-Fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester
The title compound was produced in accordance with the general method of Example 5d from (RS,RS,RS)-[3-(3-chloromethyl-4-fluoro-butyrylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester. Off-white foam, MS (ISP) 478.5 (M+H)+.
e) 1-((RS,RS,RS)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one
The title compound was produced in accordance with the general method of Example 1e from (RS,RS,RS)-[3-(4-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester. Light yellow oil, MS (ISP) 378.5 (M+H)+.
Examples 28 and 29
Examples 28 and 29
(SR)-1-((RS,RS,RS)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one
and
(RS,RS,RS,RS)-1-(2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one
The title compounds were produced from 1-((RS,RS,RS)-2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one (Example 23) by chromatographic separation (SiO2, CH2Cl2/MeOH/NH4OH 80:1:0.2, then 95:5:0.25).
(SR)-1-((RS,RS,RS)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one: Yellow oil, Rf=0.45 (CH2Cl2/MeOH/NH4OH 90:10:0.25).
(RS,RS,RS,RS)-1-(2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one: Light yellow solid, Rf=0.40 (CH2Cl2/MeOH/NH4OH 90:10:0.25).
Example 30
(S)-1-((S,S,S)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one Dihydrochloride
a) [(S,S,S)-3-(3-Chloromethyl-4-fluoro-butyrylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester
The title compound was produced in accordance with the general method of Example 5c from (S,S,S)-(3-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl)-carbamic acid tert-butyl ester (Example 16b) and 3-chloromethyl-4-fluoro-butyryl chloride (Example 23b). Off-white solid.
b) [(S,S,S)-3-((S)-4-Fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester and [(S,S,S)-3-((R)-4-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester
Sodium hydride (55–65% dispersion in oil, 1.14 g, 28.5 mmol) was added to a suspension of [(S,S,S)-3-(3-chloromethyl-4-fluoro-butyrylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester (6.72 g, 13.1 mmol) in N,N-dimethylformamide (95 mL) at r.t., then after 1 h the reaction mixture was poured onto ice and partitioned between ethyl acetate and water. The organic layer was washed with brine, dried (MgSO4), and evaporated. Chromatography (SiO2, cyclohexane/2-propanol 4:1) afforded [(S,S,S)-3-((S)-4-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester (2.40 g, 38%) and the epimer, [(S,S,S)-3-((R)-4-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester (2.73 g, 44%).
[(S,S,S)-3-((S)-4-Fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester: Light yellow foam, Rf=0.6 (SiO2, cyclohexane/2-propanol 1:1).
[(S,S,S)-3-((R)-4-Fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester: Light yellow foam, Rf=0.4 (SiO2, cyclohexane/2-propanol 1:1).
- c) (S)-1-((S,S,S)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one dihydrochloride
[(S,S,S)-3-((S)-4-Fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester (2.40 g, 5.02 mmol) was converted to (S)-1-((S,S,S)-2-amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one in accordance with the general method of Example 1e. The product was dissolved in 2-propanol (10 mL) and treated with hydrogen chloride (5–6 M in 2-propanol, 37 mL). The suspension formed was stirred for 64 h at r.t., then the precipitate was collected by filtration and dried, to afford the title compound (2.04 g, 91%). White solid, m.p. >300° C.
Example 31(R)-1-((S,S,S)-2-Amino-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-3-yl)-4-fluoromethyl-pyrrolidin-2-one dihydrochloride
The title compound was produced in accordance with the general method of Example 30c from [(S,S,S)-3-((R)-4-fluoromethyl-2-oxo-pyrrolidin-1-yl)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido [2,1-a]isoquinolin-2-yl]-carbamic acid tert-butyl ester (Example 30b). White solid, m.p. >300° C.


compd 27 (AS ABOVE) IN http://pubs.acs.org/doi/abs/10.1021/ml5001905
Imigliptin
- CAS OF FREE BASE 1314944-07-4
- C21 H24 N6 O
- Benzonitrile, 2-[[7-[(3R)-3-amino-1-piperidinyl]-2,3-dihydro-3,5-dimethyl-2-oxo-1H-imidazo[4,5-b]pyridin-1-yl]methyl]-

Sihuan Pharmaceutical
Imigliptin dihydrochloride is an orally-available dipeptidyl peptidase IV (CD26; DPP-IV; DP-IV) inhibitor in phase I clinical trials at Sihuan Pharmaceutical for the treatment of type 2 diabetes.
........................................................................
(R)-2-[[7-(3-aminopiperidin-1-yl)-3,5-dimethyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl]methyl]benzonitrile AS TFA SALT
- 1314944-08-5 CAS
- C21 H24 N6 O . C2 H F3 O2
- Benzonitrile, 2-[[7-[(3R)-3-amino-1-piperidinyl]-2,3-dihydro-3,5-dimethyl-2-oxo-1H-imidazo[4,5-b]pyridin-1-yl]methyl]-, 2,2,2-trifluoroacetate (1:1)
.....................................................................................
LEAD compd 1 as above ..........cas .........1314943-88-8
- C19 H19 N5 O2
- Benzonitrile, 2-[[7-[(3R)-3-amino-1-piperidinyl]-2-oxooxazolo[5,4-b]pyridin-1(2H)-yl]methyl]-
.............................................
SEE POLYMORPHS
CN 102863440
Dipeptidyl peptidase-IV (DPP-IV) inhibitors are a new generation of oral treatment of type 2 diabetes by enhancing the role of incretin activity, a non-insulin therapy. With conventional medicine for treating diabetes compared, DPP-IV inhibitors have not weight gain and edema and other adverse reactions. [0003] The compound shown in formula ⑴ (R) -2 - [[7 - (3 - amino-piperidine-I-yl) -3,5 - dimethyl-2 - oxo-2 ,3 - dihydro- -IH-imidazo [4,5-b] pyridin-I-yl] methyl] benzonitrile (referred to as the specification of compound A, in the patent application CN201010291056. 9 already described) is a DPP-IV inhibitor compounds , the DPP-IV has a strong inhibitory effect and high selectivity.
V
[0004] formula ⑴
[0005] In the crystalline drug development research is very important, compound crystal form, will result in its stability, solubility and other properties are different. Therefore, the inventors of the compound or its salt polymorph A lot of research carried out, whereby it was confirmed, and the invention of the compound A crystalline salt.
3, Invention
[0006] The object of the present invention is to solve the above problems and to provide better stability, better maneuverability, good bioavailability and solubility of the compound A or a salt thereof and method for preparing the crystalline form.
[0007] The present invention provides formula (I), the compound A dihydrochloride salt polymorph I: using Cu-K α radiation, to angle 2 Θ (°) represents an X-ray powder diffraction at 8. 7 ± 0. 2 °, 19.4 ± 0.2 °, 23. 5 ± 0. 2 °, 27. 2 ± 0. 2 ° at a characteristic peaks.
Butterfly NC N
[0008] formula ⑴
[0009] A compound of the dihydrochloride salt polymorph I, with Cu-Ka radiation, to angle 2 Θ (°) represents an X-ray powder diffraction peaks in addition to the features described above, it also at 12. 5 ± 0. 2 °, 22. 5 ± 0. 2 °, 25. 5 ± 0.2 ° at a characteristic peaks.
[0010] A compound of the dihydrochloride salt polymorph I, with Cu-κα exposed to radiation angle 2 Θ (°) represents an X-ray powder diffraction peaks in addition to the features described above, it also at 11.7 ± 0.2 °, 14.6 ± 0.2 °,
26. O ± 0.2 ° at a characteristic peak.
[0011] The present invention also provides the compound A dihydrochloride Preparation of polymorph I.
[0012] Compound A was dissolved in an organic solvent, and temperature, was added dropwise a stoichiometric ratio of hydrochloric acid, after the addition was complete stirring, filtered and dried to give the dihydrochloride salt of Compound A crystalline form I.
.......................................................
0r
WO 2011085643
- Diabetes mellitus is a systemic chronic metabolic disease caused by a blood glucose level higher than normal level due to loss of blood glucose control. It is basically classified into four categories, including: type I (insulin-dependent) and type II (non-insulin-dependent), the other type and gestational diabetes. Type I and type II diabetes are primary diabetes, which are the two most common forms caused by the interaction of genetic and environmental factors. The cause of diabetes is very complicated, but in the final analysis, is due to absolute or relative insulin deficiency, or insulin resistance. It is characterized by the metabolic disorder of carbohydrate, protein, fat, electrolytes and water caused by absolute or relative insulin deficiency and the reduced sensitivity of target cells to insulin.
- In recent years, because of the improvement of living level, changes in the diet structure, the increasingly intense pace of life and lifestyle of less exercise and many other factors, the global incidence of diabetes is rapidly increasing, so that diabetes has become the third chronic disease which has a serious threat to human health next to tumor and cardiovascular diseases. Presently, the number of the patients suffering from diabetes has exceeded 120 million in the world, and the number in our country is the second largest in the world. According to statistics, up to 40 million people have been diagnosed as diabetes in China, and the number of the patients is increasing at a rate of 1 million per year. Among them, patients having type I and type II diabetes accounted for 10% and 90% respectively. Diabetes has become the increasingly concerned public health issue.
- The main drugs currently used for the treatment of type I diabetes are insulin preparations and their substitutes; for the treatment of type II diabetes, the main drugs are oral hypoglycemic agents, generally divided into sulfonylureas, biguanides, traditional Chinese medicine preparations, other hypoglycemic agents, and auxiliary medication. Although these drugs have good effects, they can not maintain long-term efficacy in reducing the high blood glucose, and can not effectively alleviate the condition against the cause of diabetes. Many of the anti-diabetic drugs can well control the blood glucose at the beginning, but their efficacy can not be maintained when the treatment using such drugs are continuously used. It is one of the main reasons why combination therapies or drugs in different classes are used. However, the existing anti-diabetic drugs is lack of long-term efficacy mainly because their mechanism of action is to increase the sensitivity of target tissues to insulin action or improve insulin-producing activity of pancreas, but these drugs have no targeted effect to the reduced function of the pancreatic β cell, which is the fundamental cause of diabetes.
- Dipeptidyl peptidase-IV (DPP-IV) is widely present in the body, and is a cell surface protein involved in a variety of biological functions. It can degrade many active enzymes in vivo, such as glucagon like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), neuropeptide, substance P, and chemokines and the like. The deficiency of GLP-1 and GIP is the main cause resulting in type II diabetes (i.e., non-insulin-dependent diabetes). DPP-IV inhibitor is a new generation of anti-diabetic drug. It protects the activity of GLP-1, GIP and the like, stimulates the secretion of insulin, lowers blood glucose level by inhibiting the activity of DPP-IV, and does not cause hypoglycemia, weight gain, edema and other side effects. Its effect for lowering blood glucose level stops when a normal blood glucose level has been reached, and hypoglycemia will not occur. It can be used for a long term, and can repair the function of β-cells.
- Sitagliptin is the first marketed DPP-IV inhibitor. It rapidly became a "blockbuster" drug after marketed in 2006 by Merck. The FDA approved the saxagliptin developed by AstraZeneca and Bristol-Myers Squibb on July 31, 2009. SYR-322 developed by Takeda has an activity and selectivity better than that of sitagliptin and saxagliptin, and is currently in the phase of pre-registration. In addition, there are three drugs in clinical phase III: BI-1356 (linagliptin) developed by Boehringer Ingelheim, PF-734200 (gosogliptin) developed by Pfizer Inc, and PHX1149 (dutogliptin) developed by Phenomix Inc. Nine drugs are in the clinical phase II, and seven drugs are in clinical phase I.
- However, the limited varieties of drugs can not satisfy the clinical requirements. Accordingly, there is an urgent need for development of many DPP-IV inhibitor drugs to satisfy the clinical use.
- Example 17 The preparation of (R)-2-[[7-(3-aminopiperidin-1-yl)-3,5-dimethyl-2-oxo-2,3-dihydro-1
- -imidazo[4,5-b]pyridin-1-yl]methyl]benzonitrile (Compound 17) trifluoroacetate
(1)2,4-dichloro-6-methyl-3-nitropyridine
- 6-methyl-3-nitropyridin-2,4-diol (1.7 g, 10 mmol) was dissolved in 10 mL POCl3, heated to 95°C, and stirred for 1.5 h. The excess POCl3 was removed through centrifugation. 100 mL ice water was carefully added. The reaction solution was extracted with ethyl acetate (80 mL×3). The organic phase was combined, washed with saturated brine, dried with anhydrous Na2SO4 and spinned to dryness to afford 1.773 g yellow powder with a yield of 85.7 %.
(2) (R)-1-(2-chloro-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate
- [0216]The specific operation referred to the step (1) described in Example 1 for details. 0.96 g 2,4-dichloro-6-methyl-3-nitropyridin (4.64 mmol), and 0.933 g R-tert-butylpiperidin-3-yl-carbamate (4.66 mmol) were charged to afford 1.1 g titled product with a yield of 63.9 %.
(3) (R)-1-(2-methylamino-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate
- The specific operation referred to the step (2) described in Example 1 for details, 1.1 g (R)-1-(2-chloro-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate (2.97 mmol), and 5 mL 27 % solution of methylamine in alcohol were charged to afford 1.0 g titled product with a yield of 92.1 %.
(4) (R)-1-(2-methylamino-3-amino-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate
- The specific operation referred to the step (3) described in Example 1 for details. 1.0 g (R)-1-(2-methylamino-3-nitro-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate (2.74 mmol), and 0.1 g 10% Pd-C were charged to afford 0.873 g titled product with a yield of 95 %.
(5)(R)-1-(3,5-dimethyl-2-oxo-2,3-dihydro-1
H
- The specific operation referred to the step (4) described in Example 1 for details. 873 mg (R)-1-(2-methytamino-3-amino-6-methylpyridin-4-yl)piperidin-3-yl tert-butyl carbamate (2.60 mmol), 849 mg triphosgene (2.86 mmol), and 1.39 mL triethylamine (10.4 mmol) were charged to afford 0.813 g titled product with a yield of 86.5 %.
- -imidazo[4,5-b]pyridin-7-yl)piperidin-3-yl tert-butyl carbamate
(6)(R)-1-[1-(2-cyanobenzyl)-3,5-dimethyl-2-oxo-2,3-dihydro-1
H
- The specific operation referred to the step (5) described in Example 1 for details.813 mg (R)-1-(3,5-dimethyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-7-yl)piperidin-3-yl tert-butyl carbamate (2.25 mmol), 441 mg 2-(bromomethyl)benzonitrile (2.25 mmol), and 621 mg potassium carbonate (4.50 mmol) were charged to afford 0.757 g titled product with a yield of 70.5%.
- -imidazo[4,5-b] pyridin-7-yl]piperidin-3-yl tert-butyl carbamate
(7)(R)-2-[[7-(3-aminopiperidin-1-yl)-3,5-dimethyl-2-oxo-2,3-dibydro-1-imidazo [4,5-b]pyridin-1-yl]methyl]benzonitrile trifluoroacetate
- The specific operation referred to the step (6) described in Example 1 for details. 750 mg (R)-1-[1-(2-cyanobenzyl)-3,5-dimethyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin -7-yl]piperidin-3-yl tert-butyl carbamate (1.57 mmol), and 8.5 mL trifluoroacetic acid were charged to afford 0.680 g titled product with a yield of 88.3%.
Molecular formula: C21H24N6O Molecular weight: 376.45 Mass spectrum (M+H): 377.2
1H-NMR(D2O, 400 MHz): δ 7.64 (d, 1H), 7.42 (t, 1H), 7.29 (d, 1H), 6.93(d, 1H), 6.76(s, 1H), 5.39(d, 1H), 5.25(d, 1H), 3.27(s, 3H), 3.04(m, 1H), 2.90(m, 2H), 2.80-2.60 (m, 2H), 2.48 (m, 1H), 2.32 (s, 3H), 1.90 (m, 1H), 1.54 (m, 1H), 1.32 (m, 1H).
.........................
PAPER
We report our discovery of a novel series of potent and selective dipeptidyl peptidase IV (DPP-4) inhibitors. Starting from a lead identified by scaffold-hopping approach, our discovery and development efforts were focused on exploring structure–activity relationships, optimizing pharmacokinetic profile, improving in vitro and in vivo efficacy, and evaluating safety profile. The selected candidate, Imigliptin, is now undergoing clinical trial.
Discovery of Imigliptin, a Novel Selective DPP-4 Inhibitor for the Treatment of Type 2 Diabetes
Discovery of Imigliptin, a Novel Selective DPP-4 Inhibitor for the Treatment of Type 2 Diabetes
† Department of Project Management, Medicinal Chemistry, Process, Pharmacology, Drug Metabolism and Pharmacokenetics, Toxicology, XuanZhu Pharma, 2518 Tianchen Street, Jinan, Shandong, The People’s Republic of China
‡ School of Pharmaceutical Sciences & Institute of Human Virology, Sun Yat-Sen University, 132 East Circle Road at University City, Guangzhou, The People’s Republic of China
ACS Med. Chem. Lett., Article ASAP
DOI: 10.1021/ml5001905
data for LEAD compd 1

mono-TFA solvate (160mg, 71%).
1H NMR (d-DMSO + D2O, 600 MHz):
δ
8.01 (d, 1 H), 7.89 (d, 1 H), 7.69 (t, 1 H),
7.53 (t, 1 H), 7.40 (d, 1 H), 7.13 (d, 1 H),
5.41 (d, 1 H), 5.30 (d, 1 H), 3.25 (d, 1 H), 3.05
(m, 1 H), 2.93 (d, 1 H), 2.77 (m, 1 H),
2.65 (m, 1H), 1.95 (m, 1 H), 1.66 (m, 1 H),
1.46-1.26 (m, 2 H).
Molecular Formula C19H19N5O2:(M+H) 350.2
compd 27
mono-TFA solvate (680 mg, 88%).1H NMR (D2O, 400 MHz):δ7.64 (d, 1 H), 7.42 (t, 1 H), 7.29 (d, 1 H), 6.93(d, 1 H),
6.76 (s, 1 H), 5.39 (d, 1 H), 5.25 (d, 1 H), 3.27(s, 3 H), 3.04 (m, 1 H), 2.90 (m, 2 H),
2.80-2.60 (m, 2 H), 2.48 (m, 1 H), 2.32 (s, 3 H), 1.90 (m, 1 H), 1.54 (m, 1 H), 1.32 (m,1 H).
Molecular Formula C21H24N6O: (M+H) 377.2.
....................................................................................................

http://www.sihuanpharm.com/index.php?a=show&m=Article&id=403&l=en
Start of the first 4 volunteers in Imigliptin Dihydrochloride Phase I clinical trial
2013-10-18 16:31:08 Copyfrom: Sihuan Pharmaceutical Holdings Group Ltd.
Sihuan R&D clinical research centre (based in Beijing) announced that four healthy volunteers (human subjects) were administrated Imigliptin Dihydrochloride at first dosage of 5mg this morning around 8:00 am on 18 Oct 2013, and they all are in good conditions without any observed adverse effects so far.This is the first category 1.1 innovative drug independently developed by Sihuan Group which has now officially entered into clinical trials; that is from laboratory research into human studies. The preclinical studies of Imigliptin Dihydrochloride, a novel DPP-4 inhibitor treating type II diabetes, demonstrate excellent in vitro and in vivo activities and selectivities. In animal studies, it can protect pancreatic β–cells in long-term treatment. Pharmacokinetic studies of Imigliptin Dihydrochloride show attractive profile of good oral bioavailability, fast absorption and onset, and longer half-life compatible with the once daily dosing. We anticipate the above mentioned preclinical profiles be confirmed in our ongoing clinical trials.
.............................
http://www.google.com/patents/CN102127072A?cl=en
CN102127070A, CN102127071A,CN102741251A, EP2524917A1, US8680089,US20120289497, WO2011085643A1,WO2011085643A8,
Sitagliptin (sitagliptin) is the first one listed on the DPP-IV inhibitor, in 2006 after the listing quickly became a blockbuster for Merck. July 31, 2009, FDA has approved AstraZeneca and Bristol-Myers Squibb developed saxagliptin (saxagliptin) listed. Takeda (Taketa)'s SYR-322 activity and selectivity are superior to sitagliptin and saxagliptin, is currently in pre-registration. In addition, there are three stages of drug is in phase III: Bo Mingge Yan Gehan's BI-1356 (Iinagliptin), Pfizer's PF-734200 (gosogliptin), phenomix company PHX 1149 (dutogliptin) [0007]
In phase II drug has nine, in phase I of seven.
[0008] However, the limited varieties of drugs, can not meet the clinical needs, the urgent need to develop more of the DPP-IV inhibitor drugs to meet the clinical medication.
Example 17 (R)-2-ΓΓ7-(3 ~ amino-piperidin-yl) -3, 5_ dimethyl _2_ oxo, 3_ dihydro-IH-blind half and P "4,5 Pyridine-b1-i-a] benzonitrile Jiamou 1 (Compound 17) The system of the
[0451]
[0452] (1) 2,4 - dichloro-6 - methyl-nitropyridine _3_
[0453]
[0454] A mixture of 6 - methyl-3 - nitropyridine 2,4 - diol (1. Lg, IOmmol) dissolved in IOmL POCl3, heated to 95 ° C, stirred for 1.5 hours, rotating to excess POCl3 , ice water was added carefully IOOmL, extracted with ethyl acetate (80mLX3), the combined organic phases washed with saturated brine, dried over anhydrous Na2SO4, rotary done 1. 773g yellow powder, yield 85.7%.
[0455] (2) (R)-I-(2 - chloro-nitro _6_ _3_ _4_ picoline) piperidin-_3_ t-butyl carbamate
[0456]
[0457] Specific operation in Reference Example 1 (1), cast _ 2,4 dichloro-6 - methyl-_3_ nitropyridine 0. 96g (4. 64mmol), R-tert-butyl piperidin-_3_ yl - carbamate 0. 933g (4. 66mmol), to give the product 1. Ig, yield 63.9%.
[0458] (3) (R)-I-(2 - methylamino-nitro _6_ _3_ _4_ picoline) piperidin-_3_ t-butyl carbamate
[0459]
[0460] Specific operation in Reference Example 1 (2), cast (R) -1 - (2 - chloro-nitro _6_ picoline _3_ _4_ yl)-piperidin-3 - tert-butyl imino ester 1. Ig (2. 97mmol), 27% methylamine alcohol solution 5mL, to give the product 1. Og, yield 92.1%.
[0461] (4) (R)-I-(2 - methyl amino -3 - diamino-6 - methylpyridine _4_ yl) piperidin-_3_ t-butyl carbamate
[0462]
[0463] Specific operation in Reference Example 1 (3), cast (R)-l_ (2 - methylamino-methyl-4 _3_ nitro _6_ - yl) piperidin-3 - tert- butyl carbamate 1.0g (2. 74mmol), 10% Pd-C 0. lg, to give the product 0. 873g, 95% yield.
[0464] (5) (R)-I-(3,5 - dimethyl-2 - oxo-2 ,3 - dihydro-IH-imidazo [4,5 _b] pyridin _7_ yl)
Piperidin-3 - t-butyl carbamate
[0465]
[0466] Specific operation in Reference Example 1 (4), cast ((R)-l_ (2 - methylamino-4 _3_ methyl amino _6_ - yl) piperidin-3 - yl t-butyl carbamate 873mg (2. 60mmol), triphosgene 849mg (2. 86mmol), triethylamine 1. 39mL (10. 4mmol), to give the product 0. 813g, yield 86.5% 0
[0467] (6) (R)-l-[l_ (2 - cyano-benzyl) -3,5 _ dimethyl-2 - oxo-2 ,3 - dihydro-IH-imidazo [4, 5 -b] pyridin-7 - yl] piperidin-3 - t-butyl carbamate
[0468]
[0469] Specific operation in Reference Example 1 (5), cast (R)-I-(3,5 - dimethyl-2 - oxo-2 ,3 - dihydro-IH-imidazo [4, 5-b] pyridin-7 - yl) piperidin-3 - t-butyl carbamate 813mg (2. 25mmol), 2_ (bromomethyl) benzonitrile 441mg (2. 25mmol), potassium carbonate 621mg (4. 50mmol), to give the product 0. 757g, yield 70.5%.
[0470] (7) (R) -2 - [[7 - (3 - amino-piperidin-1 - yl) -3,5 - dimethyl-2 - oxo-2 ,3 - dihydro-IH- imidazo [4,5-b] pyridin-1 - yl] methyl] benzonitrile
[0471]
[0472] Specific operation in Reference Example 1 (6), cast (R)-l-[l_ (2 - cyano-benzyl) -3,5-dimethyl-2-_ - oxo - two H-IH-imidazo [4,5-b] pyridin-7 - yl] piperidin-3 - t-butyl carbamate 750mg (l. 57mmol), trifluoroacetic acid 8. 5mL, 0 to give the product . 680g, yield 88.3%.
[0473] MF = C21H24N6O MW: 376 * 45 MS (M + H): 377. 2
[0474] 1H-NMR (D2OdOOMHz): δ 1. 32 (1Η, m), 1. 54 (1H, m), 1. 90 (1H, m), 2. 32 (3H, s), 2. 48 (1H, m), 2. 80-2. 60 (m, 2H), 2. 90 (2H, m), 3. 04 (1H, m), 3. 27 (3H, s), 5. 25 ( 1H, d), 5. 39 (1H, d), 6. 76 (1H, s), 6. 93 (1H, d), 7. 29 (1H, d), 7. 42 (1H, t), 7. 64 (1H, d) ·
| WO2004050658A1 * | Dec 3, 2003 | Jun 17, 2004 | Boehringer Ingelheim Pharma | Novel substituted imidazo-pyridinones and imidazo-pyridazeiones, the production and use thereof as medicaments |
| WO2009099594A1 * | Feb 2, 2009 | Aug 13, 2009 | Luke W Ashcraft | Certain chemical entities, compositions and methods |
| WO2011085643A1 * | Jan 17, 2011 | Jul 21, 2011 | Kbp Biomedical Co., Ltd. | Fused pyridine derivatives |
| CN101228164A * | May 11, 2006 | Jul 23, 2008 | 布里斯托尔-迈尔斯·斯奎布公司 | Pyrrolopyridine-based inhibitors of dipeptidyl peptidase IV and methods |
6 S- SITAGLIPTIN

For description see at synfacts
Contributor: Philip Kocienski

Bao H, Bayeh L, Tambar UK * The University of Texas Southwestern Medical Center at Dallas, USA
Catalytic Enantioselective Allylic Amination of Olefins for the Synthesis of ent-Sitagliptin.
Catalytic Enantioselective Allylic Amination of Olefins for the Synthesis of ent-Sitagliptin.
Synlett 2013;
24: 2459-2463
24: 2459-2463
P. J. Kocienski
School of Chemistry
University of Leeds
Leeds LS2 9JT, UK
p.kocienski@chem.leeds.ac.uk
http://www.chem.leeds.ac.uk
School of Chemistry
University of Leeds
Leeds LS2 9JT, UK
p.kocienski@chem.leeds.ac.uk
http://www.chem.leeds.ac.uk
Philip J. Kocienski was born in Troy, New York, in 1946. His love for organic chemsitry, amply stimulated by Alfred Viola whilst an undergraduate at Northeastern University, was further developed at Brown University, where he obtained his PhD degree in 1971 under Joseph Ciabattoni. Postdoctoral study with George Büchi at MIT and later with Basil Lythgoe at Leeds University, England, confirmed his interest in the synthesis of natural products. He was appointed Brotherton Research lecturer at Leeds in 1979 and Professor of Chemistry at Southampton University in 1985. In 1990 he was appointed Glaxo Professor of Chemistry at Southampton University. He moved to the University of Glasgow in 1997, where he was Regius Professor of Chemistry and now he is a Professor of Chemistry at Leeds University.
In addition to Prof. Kocienski’s work as an author he is also a member of the SYNTHESIS Editorial Board and contributes greatly to the development of Thieme Chemistry’s journals
Furthermore, Prof. Kocienski has also contributed to the Science of Synthesis project where he was an author for Volume 4, Compounds of Group 15 (As, Sb, Bi) and Silicon Compounds.
Prof. Kocienski is also responsible for compiling a database called Synthesis Reviews. This resource is free and contains 16,257 English review articles (from journals and books) of interest to synthetic organic chemists. It covers literature from 1970 to 2002.
7 DENAGLIPTIN
DENAGLIPTIN
(2S,4S)-1-[(2S)-2- amino-3,3-bis(4-fluorophenyl)propionyl]-4-fluoropyrrolidine-2-carbonitrile, (2S,4S)-4-fluoro-1-[4-fluoro-beta-(4-fluorophenyl)-L-phenylalanyl]-2-pyrrolidinecarbonitrile
1-[2(S)-Amino-3,3-bis(4-fluorophenyl)propionyl]-4(S)-fluoropyrrolidine-2(S)-carbonitrile
GSK-823093, 823093
811432-66-3 CAS TOSYLATE
811432-66-3 CAS TOSYLATE
483369-58-0 (free base)
Denagliptin (GSK-823093) having the structural formula D below is (2S,4S)-1-[(2S)-2- amino-3,3-bis(4-fluorophenyl)propionyl]-4-fluoropyrrolidine-2-carbonitrile, also named (2S,4S)-4-fluoro-1-[4-fluoro-beta-(4-fluorophenyl)-L-phenylalanyl]-2-pyrrolidinecarbonitrile
(D) – A -
Denagliptin is specifically disclosed in US Patent No. 7,132,443 and in WO 03/002531. In one embodiment, denagliptin is in the form of its hydrochloride salt as disclosed in Example 2 of WO 03/002531 or its tosylate salt as disclosed in WO 2005/009956. A class of this embodiment refers to denagliptin tosylate. Crystalline anhydrous denagliptin tosylate is disclosed in WO 2005/009956.
Denagliptin is a dipeptidyl peptidase IV (DPP-IV) inhibitor which entered phase III clinical trials in 2006 for the treatment of type 2 diabetes at GlaxoSmithKline. Development of this compound was put on hold due to unfavorable preliminary data from preclinical long-term toxicity trials.
………………………
………………………..
Example 2
(2S,4S)-1-[(2S)-2-Amino-3,3-bis(4-fluorophenyl)propanoyl]-4-fluoropyrrolidine-2-carbonitrile hydrochloride
A. 3,3-Bis(4-fluorophenyl)-3-hydroxypropanoic acid.
To an anhydrous THF (80 mL) solution of n-butyl lithium (46 mL of 2.5 M, 115 mmol) at 0° C. was added dropwise diisopropylamine (11.13 g, 115 mmol) and the solution stirred for 10 minutes. Keeping the solution at 0° C., acetic acid (2.64 g, 44 mmol) was added dropwise and the mixture stirred for 10 min and it was then heated 50° C. After 30 min a heavy precipitate had formed and the solution was allowed to cool. A solution of 4,4′-diflurobenzophenone (9.6 g, 0.044 mol) in THF (50 mL, anhydrous) was added at 0° C., and the solution stirred at room temperature overnight. Water (100 mL) and diethyl ether (100 mL) were added and the aqueous layer was separated and acidified with 1M HCl to pH 3. The organics were extracted with ethyl acetate (3×200 mL) followed by drying over MgSO4. Filtration and removal of the solvent in vacuo yielded a crude white solid that could be washed with cold CHCl3 to remove trace amounts of the benzophenone. The solid was dried under high vacuum yielding 5.63 g (20.2 mmol, 46% yield) of compound A as a white solid.
1H NMR (d6-DMSO) 400 MHz δ 12.4 (s(br), 1H), 7.48–7.39 (m, 4H), 7.19–7.02 (m, 4H), 5.91 (s(br), 1H), 3.25 (s, 2H) ppm.
B. 3,3-Bis(4-fluorophenyl)acrylic acid.
To a 20% solution of sulfuric acid in acetic acid (50 mL, V/V) was compound A (5.6 g, 20.2 mmol) and the mixture stirred for 30 minutes at RT. To this solution was added H2O (500 mL) and the organics were extracted with ethyl acetate (3×150 mL) followed by drying over MgSO4. Filtration and removal of the solvent in vacuo yielded a white solid. The solid was dried under high vacuum yielding 4.97 g (19.1 mmol, 95% yield) of compound B as a white solid.
1H NMR (CDCl3) 400 MHz δ 7.27–7.21 (m, 2H), 7.19–7.13 (m, 2H), 7.10–6.95 (m, 4H), 6.26 (s, 1H) ppm.
C. 3,3-Bis(4-fluorophenyl)propanoic acid.
To a solution of compound B (2.5 g, 9.61 mmol) in ethyl acetate (250 mL) was added 10% palladium on carbon (50% w/w) and hydrogenated at 1 atmosphere of hydrogen for 12 hours. The heterogeneous solution was filtered through celite and concentrated in vacuo to provide a yellow oil. The oil was dried under high vacuum yielding 2.40 g (9.16 mmol, 95% yield) of compound C as a yellow oil.
1H NMR (d6-DMSO) 400 MHz δ 12.08 (brs, 1H), 7.40–7.30 (m, 4H), 7.15–7.05 (m, 4H), 4.45 (t, 1H, J=8.1 Hz), 3.05(d, 2H, J=8.1 Hz) ppm.
D. (4S,5R)-3-[3,3-Bis(4-fluorophenyl)propanoyl]-4-methyl-5-phenyl-1,3-oxazolidin-2-one.
To a THF (50 mL, anhydrous) containing compound C (2.0 g, 7.63 mmol) was added N,N-diisopropylethylamine (1.18 g, 9.16 mmol) and then the solution cooled to −78° C. To this solution was added trimethylacetyl chloride (0.97 g, 8.01 mmol) and the solution warmed to 0° C. over 1 hour. The cloudy mixture was filtered and the filtrate added slowly over 10 min to a solution of the lithiated (4S,5R)-(−)-4-methyl-5-phenyl-2-oxazolidinone at −78° C., which was prepared by the dropwise addition of n-butyl lithium (3.0 mL of 2.5 M, 7.63 mmol) to a THF (50 mL) solution of (4S,5R)-(−)-4-methyl-5-phenyl-2-oxazolidinone (1.35 g, 7.63 mmol) at −78° C. which had stirred for 10 min to provide the lithiated (4S,5R)-(−)-4-methyl-5-phenyl-2-oxazolidinone. The yellow mixture was warmed to 0° C. and quenched with H2O (50 mL) and extracted with diethyl ether (3×250 mL) followed by drying over MgSO4. Filtration and removal of the solvent in vacuo yielded a solid. Flash chromatography (silica gel, 20% ethyl acetate/hexanes) provided compound D. The white solid was dried under high vacuum yielding 2.31 g (5.49 mmol, 72% yield) as a white solid.
1H NMR (d6-DMSO) 400 MHz δ 7.40–7.25 (m, 9H), 7.18–7.02 (m, 4H), 5.76 (d, 1H, J=7.6 Hz), 4.65 (m, 1H), 4.58 (t, 1H, J=7.6 Hz), 3.72 (dd, 1H, J=16.8, 7.0 Hz) 3.57 (dd, 1H, J=16.8, 7.0 Hz), 0.58 (d, 3H, J=6.7 Hz) ppm.
E. (4S,5R)-3-[(2S)-2-Azido-3,3-bis(4-fluorophenyl)propanoyl]-4-methyl-5-[(1E,3Z)-1-methylhexa-1,3,5-trienyl]-1,3-oxazolidin-2-one.
To a THF (50 mL anhydrous) solution containing compound D (2.0 g, 4.75 mmol) at −78° C. was added dropwise potassium bis(trimethylsilyl)amide (10.0 mL of 0.5 M toluene solution, 4.98 mmol). After stirring for 10 min 2,4,6-triisopropylbenzenesulfonyl azide (trisyl azide) (1.84 g, 5.94 mmol) in THF (10 mL, anhydrous) was added in one portion. After 3 minutes acetic acid was added (1.31 g, 21.8 mmol) at −78° C. and then the reaction quickly warmed to 30° C. and stirred for 1 hr at that temperature generating a light yellow solution. To this solution was added H2O (100 mL) and the organics were extracted with ethyl acetate (500 mL). After washing with sat NaHCO3 (100 mL) and drying over MgSO4 the solvent was reomved in vacuo yielding a yellow oil. Column chromatography (ethyl acetate/hexanes 1:9) provided compound E as a white solid. HPLC showed a single diastereoisomer. The white solid was dried under high vacuum yielding 1.71 g (3.70 mmol, 78% yield) as a white solid.
1H NMR (CDCl3) 400 MHz δ 7.42–7.35 (m, H), 7.25–7.18 (m, H), 7.10–7.06 (m, 2H), 7.05–6.92 (m, 2H), 5.95 (d, 1H, J=10.8 Hz), 5.05 (d, 1H, J=7.1 Hz), 4.60 (d, 1H, J=10.8 Hz), 4.38 (m, 1H), 0.95 (d, 3H, J=6.8 Hz) ppm.
F. (2S)-2-Azido-3,3-bis(4-fluorophenyl)propanoic acid.
To a THF/H2O (4:1, 50 mL) solution of compound E (1.5 g, 3.25 mmol) at 0° C. was added a solution of lithium hydroxide (0.272 g, 6.49 mmol) in hydrogen peroxide (1.50 mL of 30% soln in H2O, 48.75 mmol). The mixture was stirred at 0° C. for 1 hr and then quenched with Na2SO4 (6.3 g, 50 mL of 1.0 M solution in H2O). The THF was removed in vacuo and the solution acidified to pH 1 with 6.0 M HCl at 0° C. The organics were extracted with ethyl acetate (2×200 mL) followed by drying over MgSO4. Filtration and removal of the solvent in vacuo yielded a clear oil. Column chromatography (EtOAc/hexanes/acetic acid 50:50:1) provided compound F as a white solid. The solid was dried under high vacuum yielding 0.78 g (2.60 mmol, 80% yield) as a white solid.
1H NMR (CDCl3) 400 MHz δ 9.60(s(br), 1H), 7.25–7.10 (m, 4H), 7.10–6.95 (m, 4H), 4.50 (d, 2H, J=8.6 Hz) ppm.
G. (2S)-2-Amino-3,3-bis(4-fluorophenyl)propanoic acid.
To an ethyl acetate (250 mL) solution of compound F (1.5 g, 4.95 mmol) was added 10% palladium on carbon (10% w/w) and hydrogenated at 1 atmosphere of hydrogen for 12 hr. The heterogeneous solution was filtered through celite (1 g) and the filtrate concentrated in vacuo to provide a clear oil. The oil was dried under high vacuum yielding 1.30 g (4.70 mmol, 95% yield) of compound G as a white solid.
1H NMR (d6-DMSO) 400 MHz δ 10.2(s(br), 1H), 7.38–7.27(m, 4H), 7.08–6.98 (m, 4H), 4.25 (d, 1H, J=8.3 Hz), 3.95 (d, 1H, J=8.3 Hz) ppm.
H. (2S)-2-[(tert-Butoxycarbonyl)amino]-3,3-bis(4-fluorophenyl)propanoic acid.
To a CH2Cl2 (150 mL) solution containing compound G (1.30 g, 4.69 mmol) was added triethylamine (2.37 g, 23.4 mmol) and di-tert-butyl dicarbonate (1.23 g, 5.63 mmol). After stirring for 12 hr H2O (50 mL) and CH2Cl2 (300 mL) were added and the solution acidified to pH 3 with 1.0 M HCl. Separation of the ethyl acetate layer followed by drying over MgSO4 and removal of the solvent in vacuo yielded a clear oil. The oil was dried under high vacuum yielding 1.68 g (4.4 mmol, 95% yield) of compound H as a white solid.
1H NMR (d6-DMSO) 400 MHz δ 12.4 (s(br), 1H), 7.35–7.22 (m, 4H), 7.15–6.95 (m, 4H), 4.78 (t, 1H, J=8.9 Hz), 4.25 (d, 1H, J=8.9 Hz), 3.05 (m, 1H), 1.20 (s, 3H), 1.15 (s, 6H) ppm.
I. (2S,4S)-1-[(2S)-2-(tert-Butoxycarbonyl)amino-3,3-bis(4-fluorophenyl)propanoyl]-4-fluoropyrrolidine-2-carbonitrile.
To a DMF solution (25 mL anhydrous) was compound H (1.0 g, 2.65 mmol) and HATU (1.0 g, 2.65 mmol). To this solution was added N,N-diisopropylethylamine (0.462 mL, 2.65 mmol) and after 30 min (2S, 4S)-4-fluoro-2-pyrrolidinecarbonitrile 4-methylbenzenesulfonate (0.619 g, 2.12 mmol) and additional N,N-diisopropylethylamine (0.37 mL 2.12 mmol) were added. This solution was allowed to stir at RT for 12 hr and then saturated sodium bicarbonate (100 mL) was added. The resulting gummy mixture was extracted with ethyl acetate (3×100 mL) and the organics were washed with saturated NaCl (50 mL) followed by drying over MgSO4. Filtration and removal of the solvent in vacuo yielded a clear oil. The oil was chromatographed on silica gel (hexanes/EtOAc 4:1) to provide a white solid. The solid was dried under high vacuum yielding 815 mg (1.72 mmol, 65% yield) of compound I as a white solid.
1H NMR (CDCl3) 400 MHz δ 7.38–7.32 (m, 2H), 7.21–7.15 (m, 2H), 7.12–6.98(m, 4H), 5.15 (d, 1H, J=51 Hz), 5.03 (d, 1H, J=8.9 Hz, 4.89 (d, 1H, J=11.2 Hz), 4.86 (d, 1H, J=8.9 Hz), 4.40 (d, 1H, J=11.2 Hz), 3.83 (ddd, 1H, J=36.8, 12.1, 3.7 Hz), 3.05 (d, 1H, J=12.2 Hz), 2.62 (t, 1H, J=15.3 Hz), 2.25 (m, 1H), 1.38 (s, 9H) ppm.
J. (2S,4S)-1-[(2S)-2-Amino-3,3-bis(4-fluorophenyl)propanoyl]-4-fluoropyrrolidine-2-carbonitrile hydrochloride.
To compound I (0.5 g, 1.05 mmol) was added 4.0 N HCl in 1,4-dioxane (10 mL, 40 mmol) and after 3 hr diethyl ether (100 mL) was added. The resulting precipitate was collected by filtration and after drying under high vacuum 0.41 g (1.0 mmol, 95% yield) of compound J was obtained as a white solid.
1H NMR (d6-DMSO) 400 MHz δ 8.42 (s(br), 3H), 7.72–7.66 (m, 2H), 7.38–7.32 (m, 2H), 7.25–7.19 (m, 2H), 7.06–7.0 (m, 2H), 5.38 (d, 1H, J=51 Hz), 4.91 (d, 2H, J=8.8 Hz), 4.82 (d, 1H, J=11.3 Hz), 4.41 (d, 1H, J=11.3 Hz), 3.86 (ddd, 1H, J=39.2, 12.4, 3.1 Hz), 3.45 (q, 1H, J=12.4 Hz), 2.38–2.20 (m, 2H) ppm.
…………………
PAPER
Org. Process Res. Dev., 2009, 13 (5), pp 900–906
DOI: 10.1021/op900178d
A recent paper from workers at GSK describes improvements to the synthesis of Denagliptin (12). The final chemical step is Boc deprotection of (11) with p-toluenesulphonic acid (p-TSA) in isopropanol (IPA). Some isolated batches of final product contained impurities 12A (~1%), 12B (~1%), and 12C (~0.3%). Investigation showed that these three impurities were not produced during the reaction but were produced in the dryer if there was any excess p-TSA in the filter cake during drying. These impurities could be avoided by washing the filter cake with 2 volumes of IPA prior to drying.
D.E. Paterson,* J.D. Powers, M. LeBlanc, T. Sharkey, E. Boehler, E. Irdam, and M.H. Osterhout (GlaxoSmithKline), Org. Process. Res. Dev.,2009, 13(5), 900-906.
Denagliptin Tosylate (1)
To a mixture of 11 (110 kg, 232 mmol) in isopropanol (550 L, 5 vol) at 70 °C was added a solution of p-toluenesulfonic acid monohydrate (88.4 kg, 464 mol) in isopropanol (550 L, 5 vol) over one hour while maintaining the temperature at 70 °C. After the addition, the reaction was stirred at 70 °C for 6 h. The batch was cooled to 20 °C, held for 30 min, filtered, and washed with isopropanol (2 × 220 L, 2 vol). The solids were dried at 55 °C to give 118 kg (89%) of 1 as a white solid.
Recrystallization of Denagliptin Tosylate (1)
A mixture of denagliptin tosylate (100 kg, 183 mol) and isopropanol (500 L, 5 vol) and water (500 L, 5 vol), was heated until all the solids dissolved (approximately 72 °C). The hot solution was filtered into another vessel. The solution was cooled to approximately 5 °C, and water (300 L, 3 vol) was added. The reaction was stirred at this temperature for 30 min and was filtered. The filtercake was washed with filtered isopropanol (2 × 200 L, 2 × 2 vol), and pulled dry. The solids were dried at 55 °C to give 91.9 kg (92%) of 1as a white solid.
…………………………….
(2S,4S)-4-fluoro-1-[4-fluoro-β-(4-fluorophenyl)-L-phenylalanyl]-2-pyrrolidinecarbonitrile p-toluenesulfonic acid salt
EXAMPLE 1Preparation of (2S,4S)-4-fluoro-1-[4-fluoro-β-(4-fluorophenyl)-L-phenylalanyl]-2-pyrrolidinecarbonitrile p-toluenesulfonic acid salt, Form 1a) Preparation of (4S)-1-(tert-butoxycarbonyl)-4-fluoro-L-prolinamide
A reactor was charged with (4S)-1-(tert-butoxycarbonyl)-4-fluoro-L-proline (130 g, 1 wt, 1 eq.), dichloromethane (520 mL, 4 vol), pyridine (55 mL, 0.4 vol, 1.2 eq), and Boc-anhydride (145 g, 1.1 wt, 1.2 eq.). The reaction solution was stirred at approximately 20° C. for 2 hours. The reactor was charged with ammonium bicarbonate (62 g, 0.5 wt, 1.44 eq), and was stirred at approximately 20° C. overnight. The reaction was filtered over a bed of celite (130 g, 1 wt), and the filter cake was washed with dichloromethane (260 mL, 2 vol). The filtrate was concentrated to a volume of 3 volumes, heptane (520 mL, 4 vol) was added, and again concentrated to a final volume of 3 volumes. Heptane (390 mL, 3 vol) was added, and the reaction was cooled to approx. 5° C. for 30 min.
The solid was collected by filtration, washed with heptane (260 mL, 2 vol), and then dried under vacuum at approximately 50° C. to constant weight. Yield: 88-90%.
b) Preparation of (2S,4S)-4-fluoropyrrolidine-2-carbonitrile para-toluenesulfonic acid
The reactor was charged with (4S)-1-(tert-butoxycarbonyl)-4-fluoro-L-prolinamide (116 g, 1 wt, 1 eq.), isopropyl acetate (578 mL, 5 vol), and pyridine (88 mL, 0.8 vol, 2.2 eq). The resulting slurry was stirred at approx. 20° C. Trifluoroacetic anhydride (77 mL, 1.0 wt, 1.1 eq.) was added over at least 30 minutes, maintaining the temperature at approx. 20° C. The reaction solution was stirred an additonal 1 hour at approx. 20° C. Water (578 mL, 5 vol) was added slowly, and the reaction mixture was stirred for 15 minutes. The stirring was stopped, the layers were allowed to separate, and the aqueous (lower) layer was discarded. The organic layer was concentrated under vacuum at a jacket temperature of approximately 50° C. to half volume. The reaction was diluted back up to 5 volumes with isopropyl acetate. The reactor contents were cooled to 20° C., and the reactor was charged with p-toluenesulfonic acid (94 g, 0.8 wt, 1 eq). The reaction was stirred for 2 hours, and GC analysis at this point should show complete consumption of (4S)-1-(tert-butoxycarbonyl)-4-fluoro-L-prolinamide. The reaction was concentrated to 3 volumes under full vacuum at a jacket temperature of approximately 50° C. and 2 volumes of isopropyl alcohol were added. The reaction was concentrated to a final volume of 4 volumes. The reaction was cooled to 0° C. and held for 30 minutes. The solids were collected by filtration, washed with isopropyl alcohol (1 vol), and then dried under vacuum at approx. 50° C. to constant weight. Yield: 68-71%.
c) Preparation of tert-Butyl{(1S)-1-[bis(4-fluorophenyl)methyl]-2-[(2S,4S)-2-cyano-4-fluoro-1-pyrrolidinyl]-2-oxoethyl}carbamate
A reactor was charged with N-{[(1,1-dimethylethyl)oxy]carbonyl}-4-fluoro-β-(4-fluorophenyl)-L-phenylalanine (400 g, 1 wt, 1 eq.), (2S,4S)-4-fluoropyrrolidine-2-carbonitrile para-toluenesulfonic acid (307.7 g, 0.77 wt, 1.01 eq.), O-(7-Azabenzotriazol-1-yl)-N,N,N,N-tetramethyluronium hexaflurophosphate [i.e. HATU] (408 g, 1.02 wt, 1.01 equiv.), and DMF (2.8L, 7 vol). The mixture was cooled to approximately 0° C. Hunig’s base (376 mL, 0.94 vol, 2.04 equiv.) was added over at least 30 minutes. The mixture was heated to approximately 25° C. and was stirred at this temperature until the reaction was complete (ca. 3 hours). MTBE (2.8L mL, 7 vol) was added, followed by water (2L, 5 vol) over at least 30 minutes to quench the reaction. The aqueous phase was extracted with MTBE (1.2L, 3 vol). The combined organic phases were washed with water (2L, 5 vol). The organic phase was concentrated under vacuum to 3 volumes, and ethanol (1.6L, 4 vol) was added. The reaction was further concentrated under vacuum to 3 volumes, and ethanol (1.6 L, 4 vol) was added. The reaction was further concentrated under vacuum to 3 volumes. Added ethanol (2L, 5 vol). The ethanol solution of tert-Butyl {(1 S)-1-[bis(4-fluorophenyl)methyl]-2-[(2S,4S)-2-cyano-4-fluoro-1-pyrrolidinyl]-2-oxoethyl}carbamatewas used directly in the next step.
d) Preparation of (2S,4S)-4-fluoro-1-[4-fluoro-β-(4-fluorophenyl)-L-phenylalanyl]-2-pyrrolidinecarbonitrile p-toluenesulfonic acid salt. Form 1
A 10L reactor equipped with overhead stirring was charged with a slurry of tert-Butyl {(1S)-1-[bis(4-fluorophenyl)methyl]-2-[(2S,4S)-2-cyano-4-fluoro-1-pyrrolidinyl]-2-oxoethyl}carbamate (500 g, 1 wt, 1 eq) in ethanol (3.5L, 7 vol). To this solution was added para-toluenesulfonic acid (403g, 0.806 wt, 2 eq). This solution was heated to 60° C., and was allowed to stir at this temperature for 12 hours. The reaction mixture was cooled to 5° C. and was stirred at this temperature for 30 minutes. The solids were collected by filtration, washed with ethanol (2×1 L), and dried to constant weight in a 50° C. vacuum oven. Yield: 70-80% over 2 steps.
………………….
Augustyns, K. et al., “The Unique Properties of Dipeptidyl-Peptidase IV (DPP IV/CD26) and the Therapeutic Potential of DPP IV Inhibitors,” Current Medicinal Chemistry, V6, N4, 1999, pp. 311-327.
| US7132443 * | 26 Jun 2002 | 7 Nov 2006 | Smithklinebeecham Corporation | Fluoropyrrolidines as dipeptidyl peptidase inhibitors |
| WO2003002531A2 | 26 Jun 2002 | 9 Jan 2003 | Curt Dale Haffner | Fluoropyrrolidines as dipeptidyl peptidase inhibitors |
……………………..
8
DUTOGLIPTIN
Dutogliptin tartrate
Syn name: 1-[N-[3(R)-Pyrrolidinyl]glycyl]pyrrolidin-2(R)-ylboronic acid L-tartrate
Cas number: 890402-81-0
Molecular Formula: C14H26BN3O9
Molecular Weight: 391.18
Syn name: 1-[N-[3(R)-Pyrrolidinyl]glycyl]pyrrolidin-2(R)-ylboronic acid L-tartrate
Cas number: 890402-81-0
Molecular Formula: C14H26BN3O9
Molecular Weight: 391.18
[1-[2-(Pyrrolidin-3-ylamino)acetyl]pyrrolidin-2-yl]boronic Acid; [(2R)-1-[2-[[(3R)-Pyrrolidin-3-yl]amino]acetyl]pyrrolidin-2-yl]boronic acid
C10H20BN3O3, 241.0951
852329-66-9
- Dutogliptin
- PHX1149
- UNII-38EAO245ZX
clinical trials
PHX-1149 is a dipeptidyl peptidase IV (CD26; DPP-IV; DP-IV) inhibitor which had been in phase III clinical trials at Phenomix and Forest for the oral, once-daily treatment of type 2 diabetes.
In 2008, the compound was licensed to Forest by Phenomix in North America for development and commercialization; however this license agreement was terminated in 2010. In 2009, the compound was licensed to Chiesi by Phenomix for development and commercialization for the treatment of diabetes type 2 in Europe, Brazil, the Russian Federation and all other members of the Commonwealth of Independent States, Turkey and Northern Africa. Phenomix ceased operations in 2010.
………………………….
or
The enzyme dipeptidyl peptidase IV (DPP-IV) is a member of the dipeptidyl peptidase family, which cleaves N-terminal dipeptide residues from proteins, particularly where the dipeptide includes an N-terminal penultimate proline or alanine residue. DPP-IV is believed to be involved in glucose control, as its peptidolytic action inactivates the insulotropic peptides glucagon-like peptide I (GLP-I) and gastric inhibitory protein (GIP).
Inhibition of DPP- IV, such as with synthetic inhibitors in vivo, can serve to increase plasma concentrations of GLP-I and GIP, and thus improve glycemic control in the body. Such synthetic inhibitors would therefore be useful in the treatment of diabetes mellitus and related conditions. Certain such selective DPP-IV inhibitors have been developed, as are disclosed in U.S. Patent 7,317,109, U.S. Patent 7,576,121, U.S. Application Publication Nos. 2007/0060547, 2007/0185061, 2007/0299036, 2008/0182995, 2008/0300413, 2006/0264400, and 2006/0264401, and in International Applications WO2008/027273 and WO2008/144730, the contents of which are incorporated herein by reference. Inhibition of DPP-IV by compounds of the structure of formula (I) is disclosed therein:
Example 1 – Synthesis of (R)-N-( 1 , 1 -Dimethylethoxycarbonyl)(pyrrolidine-2-yl)boronic Acid.
An oven dried 1 L three neck round bottom flask equipped with an overhead stirrer, addition funnel and internal thermocouple was charged with (IS, 2S)-Dimethyl-bis(3,3- dimethylbutyl)cyclohexane-l,2-diamine (approx. 50 g, 161.23 mmol, 1.2 eq), BOC-pyrrolidine (approx. 23.55 ml, 134.35 mmol, 1 eq) and dry toluene (approx. 500 ml) under inert atmosphere. The clear colorless solution was cooled to “ 78° C and a solution of sec-BuLi (approx. 115.16 ml of a 1.4 solution in cyclohexane, 161.23 mmol, 1.2 eq) was added slowly via dropping funnel over approx. 10 minutes (the temperature of the reaction mixture was maintained between approx. – 780C and -650C). The light orange colored solution was stirred for 3.5 hours at approx. -780C, which was then followed by the addition of a solution of trimethylborate (approx. 45.06 ml, 403.05 mmol, 3 eq) in toluene (approx. 75 ml) via dropping funnel over 30 minutes while maintaining the temperature below -650C. The reaction mixture was warmed slowly to room temperature, and stirred for 16 hours at room temperature. The reaction mixture was added into an aqueous sodium hydroxide solution (approx. 670 ml of 2.0 M solution, 1340 mmol, 10 eq) and the resulting cloudy mixture was stirred for 30 minutes before allowing layers to separate. The aqueous phase (product) was transferred to a receiver and backwashed with toluene (approx. 100 ml). The organic phases (chiral amine ligand) were transferred to a receiver for later isolation. The aqueous phase was acidified to pH 5-6 by slow addition of HCl {cone), then extracted with EtOAc (approx. 3 x 500 ml). The organic extracts were combined, dried over Na2SO4 and concentrated until a final volume of approximately 100 ml. Heptane (approx. 300 ml) was added and the concentrated mixture was stirred at room temperature overnight (approx. 15 hours). The resulting white precipitate was filtered and the filter cake was washed with cold heptane. The product was dried at room temperature under vacuum to yield (R)- (pyrrolidine-2-yl)boronic acid (approx. 20.31 g, 94.44 mmol, 70.27 %) as a white solid. [α]25D – 72.5 (c 1, DCM); 94-95 % ee (% ee was determined through chiral HPLC); 1H NMR (400 MHz, D2O) δ 3.40-3.50 (IH), 3.20- 3.30 (IH), 2.90-3.00 (IH), 2.10 (IH), 2.00 (IH), 1.85 (IH), 1.72 (IH), 1.45-1.48 (9H); m/z (ES+) 216.06.
Example 2 – Isolation of the chiral ligand ((1S, 2S)-Dimethyl-bis(3,3-dimethyl butyl) cyclohexane- 1 ,2-diamine)
Water (approx. 300 ml) was added to the first organic extract from the previous workup and cooled to 0° C the mixture was acidified to pH 3 by slow addition of HCl. The resulting cloudy mixture was stirred vigorously before allowing layers to separate. The aqueous phase (product) was transferred to a receiver and backwashed with toluene (approx. 100 ml). The aqueous phase was stirred at O0C and the pH of the solution was adjusted to 12-13 by the addition of sodium hydroxide. The mixture was extracted with toluene (approx. 3 x 500 ml) and the combined organic phases were concentrated under reduced pressure to give the crude chiral diamine (approx. 48.32 g, 155.57 mmol, 96.5%) as light yellow oil. Further purification by vacuum distillation (approx. 120-1300C, house vacuum) yielded the chiral diamine as a colorless oil (approx. 45.57 g, 146.72 mmol) in 91% recovery).Example 3 – Synthesis of (R)-N-(I, l-dimethylethoxycarbonyl)-pinanediol-(Pyrrolidin-2-yl) boronate
A solution of (R)-Pyrrolidine boronic acid (approx. 300 mg, 1.39 mmol) in isopropyl acetate (approx. 10 ml) was treated with (+)-pinanediol (approx. 236.35 mg, 1.39 mmol, 1 eq) and Na2SO4 (approx. 203.25 mg, 1.39 mmol, 1 eq). After 24 hr, the solvent was evaporated to give crude boronic ester (approx. 475.55 mg, 1.36 mmol, 98 %) as a clear oil: 98-99 % de via chiral HPLC; 1U NMR (400 MHz, CDCl3) δ 4.32 (IH), 3.47 (IH), 3.41-3.31 (2H), 3.22-3.05 (IH), 2.38- 2.30 (IH), 2.20-1.75 (8H), 1.45 (9H), 1.41 (3H), 1.28 (3H), .85 (3H); m/z (ES, M+l) 350.28.Example 4 – (R)-N-(Pyrrolidine-2-yl)-pinacol boronate
To a solution of pyrrolidine boronic acid (approx. 456 mg, 2.12 mmol) in isopropyl acetate
(approx. 15 ml) was added pinacol (approx. 251 mg, 2.12 mmol, 1 eq) and Na2SO4 (approx. 310 mg, 2.12 mmol, 1 eq). The mixture was stirred for 24 hr and the solvent was evaporated to yield crude pinacol boronate. The residue was triturated with EtOAc/hexane (approx. 1 : 10) at RT for 1 hr then filtered to give the pinacol boronate (approx. 611 mg, 2.06 mmol, 97 %) as a white solid: . 1H NMR (400 MHz, CDCl3) δ 3.40-2.95 (3H), 1.95-1.50 (4H), 1.40 (9H), 1.20 (12H); m/z (ES+) 298.21. Removal of the Boc-protecting group was achieved by dissolving the white solid pinacol boronate in dry ether (approx. 15 ml), cooling to 0° C in an ice bath followed with addition of 1.5 eq of HCl in dioxane After 8 hours, the solvent was evaporated then triturated in hexane for 1 hr. The white precipitate was filtered and dried to yield the acid salt (approx. 472 mg, 2.02 mmol, 98 %): 1HNMR (CDCl3) δ 3.48 (IH), 3.36 (IH), 3.21 (IH), 2.21 (IH), 2.03 (2H), 1.95 (IH), 1.35 (12H); m/z (ES M+l) 198.21.
Example 5 – Synthesis of (R)-3-(Benzyloxycarbonyl-{2-oxo-2-[(R)-2-((lS,2S,6R,8S)-2,9,9- trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.0^'”]dec-4-yl)-pyrrolidin-l-yl]-ethyl}-amino)- pyrrolidine- 1-carboxylic acid benzyl ester
A mixture of (R)-3-(benzyloxycarbonyl-carboxymethyl-amino)-pyrrolidine- 1-carboxylic acid benzyl ester dicyclohexylamine salt) (approx. 300.Og, 0.505mol), water (approx. 1.5L), 2M aqueous sulfuric acid (approx. 0.75L, 1.5mol) and toluene (approx. 2L) was stirred in a 1OL reactor at room temperature for 15 min. After settling the layers were separated. The aqueous layer was stirred with toluene (approx. 1.0L) for 15 min, and the layers were separated. The combined organic layers were washed with water (approx. 1.5L), and concentrated under vacuum at 450C to 1.5L. To this solution was added N-methylmorpholine (approx. 55.4 mL, 0.505mol) and this mixture was added to a cold solution (approx. 0°-5°C) of ethyl chloroformate (approx. 48.1 mL, 0.505mol) in toluene (approx. 1.0L). The reaction mixture was stirred at 0° – 50C for 15 min and solid (2-(2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02‘6]dec-4-yl)-pyrrolidine hydrochloride) (approx. 144.4g, 0.505mol) was added in one portion followed by addition of N- Methylmorpholine (approx. 110.8 mL, l.Olmol). The mixture was stirred for 30 min at 0°-5°C, and allowed to warm to 20°-25°C. Stirring was continued for an additional 2.5 h. Water (approx. 2.0L) was then added, and the mixture was stirred for an additional 15 min. The layers were separated and the organic layer was subsequently washed with 0.85M aqueous sodium bicarbonate solution (approx. 1.2L), water (approx. 2.0L), and 0.065M citric acid solution (approx. 1.5L). Toluene solution was concentrated under vacuum at 450C, to give 287.3 g (approx. 88.4%) of the title compound. 1H NMR (400 MHz, CDCl3, ppm): mixture of rotomers, 7.35-7.25 (10H,m); 5.22- 4.99 (4H,m); 4.60 (IH, d); 4.22 (IH, dd); 4.11-3.65 (3H, m); 3.60-3.00 (6H, m); 2.32-1.91 (8H, m); 1.89-1.67 (4H, m); 1.42-1.18 (6H, m); 0.84-0.72 (3H, m); m/z (M+H)=644. Example 6 – Synthesis of 2-((R)-Pyrrolidin-3-ylamino)-l-[(R)-2-((lS,2S,6R,8S)-2,9,9-trimethyl- 3,5-dioxa-4-bora-tricyclo[6.1.1.0 ‘ ]dec-4-yl)-pyrrolidin- 1 -yl]-ethanone
a) THF solvateA solution of (R)-3-(Benzyloxycarbonyl-{2-oxo-2-[(R)-2-((l S,2S,6R,8S)-2,9,9-trimethyl-3,5- dioxa-4-bora-tricyclo[6.1.1.02‘”]dec-4-yl)-pyrrolidin- 1 -yl] -ethyl }-amino)-pyrrolidine- 1 – carboxylic acid benzyl ester (approx. 4.76 g, 7.4 mmol) in toluene (approx. 60 mL) was diluted with methanol (approx. 60 mL). 10% Pd/C (wet, 500 mg) was added, and the mixture was hydrogenated at 50 psi for 3 h. The mixture was filtered through celite and washed with methanol (approx. 10 mL). The solution was then concentrated under vacuum to dryness. The residue was dissolved in THF (approx. 10 mL) at 4O0C and crystallized overnight at -1O0C to -15°C. Crystals were filtered, washed with cold THF (approx. 3 mL), and dried under vacuum for 5 h to yield 1.9 g (approx. 68.5%) of the title compound. 1H NMR (400 MHz, D2O, 1 drop TFA), 64.18 – 4.89 (m, IH), 3.93 – 3.85 (m, IH), 3.77 (s, 2H), 3.55 (dd, IH)5 3.45 -3.38 (m, 4H), 3.35 – 3.25 (m, 2H), 3.24 – 3.05 (m, 3H), 2.93 (t, IH), 2.33 – 2.24 (m, IH), 2.15 – 1.42 (m, 16H), 1.09 (s, 3H), 0.94 (s, 3H), 0.78 (d, IH), 0.50 (s, 3H). m/z (ES+) = 376.30.
Thermogravimetric analysis of THF solvate of 2-((R)-Pyrrolidin-3-ylamino)-l-[(R)-2-
((lS,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02‘6]dec-4-yl)-pyrrolidin-l-yl]- ethanone was performed as is shown in Figure 5.
X-Ray Diffractogram of THF solvate of 2-((R)-Pyrrolidin-3-ylamino)-l-[(R)-2-((lS,2S,6R,8S)- 2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02‘6]dec-4-yl)-pyrrolidin-l-yl]-ethanone was performed as is shown in Figure 6. b) Non-solvate
A solution of (3-(Benzyloxycarbonyl-{2-oxo-2-[2-(2,9,9-trimethyl-3,5-dioxa-4-bora- tricyclo[6.1.1.02‘6]dec-4-yl)-pyrrolidin-l-yl]-ethyl}-amino]-pyrrolidine-l-carboxylic acid benzyl ester) (approx. 20.Og, 31.Ommol) in toluene (approx. 8OmL) was diluted with methanol (approx. 20 mL). 10% Pd/C (2g, wet) was added, and the mixture was hydrogenated at 50 psi for 3 h. The mixture was filtered through celite and the filter bed was washed with a mixture of toluene (approx. 2OmL) and methanol (approx. 4 mL). The solution was concentrated to 8OmL at 30 -35 0C under vacuum (approx. 90 to 120 mBar). THF (approx. 10OmL) was added and the solution was concentrated to 12OmL at 30 -35 0C under vacuum (approx. 90 to 120 mBar). The mixture was stirred at 35 0C for Ih, resulting in crystallization. The mixture was cooled to 0 0C and held at that temperature for 2h. Crystals were isolated by filtration, washed with a cold mixture of toluene (approx. 20 mL) and THF (approx. 5 mL), and dried under vacuum at 35 0C for 16 h to yield 9.11 g (approx. 24.3 mmol, 78%) of the title compound as a white solid.1H NMR (400 MHz, D20, 1 drop TFA), δ 4.34 (dd, IH, J= 9, 2 Hz), 4.08 (m, IH), 3.99 (s, 2H), 3.74 (dd, IH, J= 13, 8 Hz), 3.52 -3.29 (m, 6H), 3.12 (t, IH, J= 8 Hz), 2.47 (m, IH), 2.27 (m, IH), 2.19 – 2.06 (m, 2H), 2.02 – 1.84 (m, 6H), 1.67 (m, 2H), 1.30 (s, 3H), 1.15 (s, 3H), 1.00 (d, IH, J= 11 Hz), 0.71 (s, 3H). m/z (ES+) = 376.30.
Thermogravimetric analysis of 2-((R)-Pyrrolidin-3-ylamino)-l-[(R)-2-((lS,2S,6R,8S)-2,9,9- trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.0^'”]dec-4-yl)-pyrrolidin-l-yl]-ethanone was performed as is shown in Figure 7.
X-Ray Diffractogram of2-((R)-Pyrrolidin-3-ylamino)-l-[(R)-2-((lS,2S,6R,8S)-2,9,9-trimethyl-
3,5-dioxa-4-bora-tricyclo[6.1.1.0 ‘ ]dec-4-yl)-pyrrolidin-l-yl]-ethanone was performed as is shown in Figure 8.
Example 7 – Synthesis of Dutogliptin Tartrate
A round bottom flask equipped with a magnetic stirrer was charged with 2-(Pyrrolidin-3- ylamino)- 1 -[2-(2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0]dec-4-yl)-pyrrolidin-l-yl]- ethanone (approx. l:l-Pinanediol borane / THF complex; 2.98 g, 6.67 mmol, leq), (L)-tartaric acid (approx. 1.00 g, 6.67 mmol, 1 eq), and H2O (approx. 15 mL). The mixture was allowed to stir for 1 hour then tert-Butyl methyl ether (approx. 15 ml) and (i?)-N-(l,l- dimethylethoxycarbonyl)(pyrrolidine-2-yl)boronic acid (approx. 1.46 g, 6.80 mmol, 1.02 eq) were added. The bi-phasic mixture was allowed to stir for 20 hours at room temperature before separating the layers. The aqueous phase backwashed with tert-butyl methyl ether (approx. 15 ml) and the organic layers were combined. Lyophilization of the aqueous layer provided dutogliptin tartrate as a white solid (approx. 2.60 g, 6.65 mmol, 99.7%): 1H NMR (400 MHz, D2O, one drop of TFA) δ 4.48 (2H), 3.95-3.88 (IH), 3.81 (2H), 3.59-3.54 (IH), 3.37-3.28 (2H), 3.21-3.16 (2H), 3.11-3.07 (IH), 2.82-2.78 (IH), 2.37-2.28 (IH), 2.04-1.96 (IH), 1.88-1.78 (2H), 1.71-1.60 (IH), 1.50-1.42 (IH); m/z (ES+) 241.10 (-tartrate acid).
| US20060069250* | Sep 28, 2005 | Mar 30, 2006 | Xiaohu Deng | Synthesis by chiral diamine-mediated asymmetric alkylation |
| US20080182995* | Oct 31, 2007 | Jul 31, 2008 | Phenomix Corporation | Pyrrolidine compounds and methods for selective inhibition of dipeptidyl peptidase-iv |
| US20080300413* | Jul 27, 2006 | Dec 4, 2008 | David Alan Campbell | Efficiently preparing boropyrrolidines and derivatives by coupling a (pyrrolidin3-yl-amino-)acetic acid and a 7,9,8-dioxaborotricyclic- (4,3,0,1(2,4))decane; protecting groups avert side reactions; antidiabetic agents |
http://organicsynthesisinternational.blogspot.in/p/gliptin-series-22.html
see gliptins at............http://drugsynthesisint.blogspot.in/p/gliptin-series.html
.........................
PART 2 AT.......http://organicsynthesisinternational.blogspot.in/p/gliptin-series-22.html
Drugs belonging to this class are :
- Sitagliptin[5] (FDA approved 2006, marketed by Merck & Co. as Januvia),
- Vildagliptin[6] (EU approved 2007, marketed in the EU by Novartis as Galvus),
- Saxagliptin (FDA approved in 2009, marketed as Onglyza),
- Linagliptin (FDA approved in 2011, marketed as Trajenta by Eli Lilly Co and Boehringer Ingelheim),[7]
- Anagliptin (approved in Japan in 2012, marketed by Sanwa Kagaku Kenkyusho Co., Ltd. and Kowa Company, Ltd.)[8]
- Teneligliptin (approved in Japan in 2012[9])
- Alogliptin (FDA approved 2013, marketed by Takeda Pharmaceutical Company)
- Gemigliptin (being developed by LG Life Sciences)[10]
- Dutogliptin (being developed by Phenomix Corporation), Phase III[11]













































This is such nice and useful information for us. I really appreciate this blog to have this kind of knowledge and it is good to know the latest updates. Also explore Potassium Carbonate Suppliers
ReplyDelete