1 QUINALAPRIL
2 BENAZEPRIL
3
4
will be updated
Enalapril (Vasotec/Renitec®); Ramipril (Altace/Tritace/Ramace Ramiwin®); Quinapril (Accupril®), Perindopril (Coversyl/Aceon®); Lisinopril (Lisodur/Lopril/Novatec Prinivil/Zestril®) and Benazepril (Lotensin®);
.........................................................................
1



.........................................................................
2







2 BENAZEPRIL
3
4
will be updated
Enalapril (Vasotec/Renitec®); Ramipril (Altace/Tritace/Ramace Ramiwin®); Quinapril (Accupril®), Perindopril (Coversyl/Aceon®); Lisinopril (Lisodur/Lopril/Novatec Prinivil/Zestril®) and Benazepril (Lotensin®);
.........................................................................
1
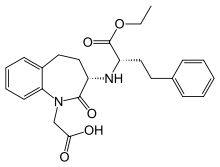
QUINAPRIL

QUINAPRIL HYDROCHLORIDE
Quinapril (marketed under the brand name Accupril by Pfizer) is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used in the treatment of hypertension andcongestive heart failure.
Quinapril inhibits angiotensin converting enzyme, an enzyme which catalyses the formation of angiotensin II from its precursor, angiotensin I. Angiotensin II is a powerfulvasoconstrictor and increases blood pressure through a variety of mechanisms. Due to reduced angiotensin production, plasma concentrations of aldosterone are also reduced, resulting in increased excretion of sodium in the urine and increased concentrations ofpotassium in the blood.
-
The condensation of alanine tert-butyl ester (I) with ethyl 2-bromo-4-phenylbutanoate (II) by means of triethylamine in hot DMF gives ethyl 2-[[1-(tert-butoxycarbonyl)ethyl]amino]-4-phenylbutanoate (III), which is partially hydrolyzed with trifluoroacetic acid yielding ethyl 2-[[1-carboxyethyl]amino]-4-phenylbutanoate (IV). The condensation of (IV) with tert-butyl-1,2,3,4-tetrahydroisoquinoline-3-carboxylate (VIII) [prepared from the corresponding acid (VI) and isobutylene (B) by means of H2SO4] as before gives tert-butyl-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylate (IX), which is finally hydrolyzed partially by treatment with trifluoroacetic acid.
 Hoefle, M.L.; Klutchko, S. (Pfizer Inc.); Substituted acyl derivatives of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids. DD 201787; EP 0049605; EP 0096157; US 4344949
Hoefle, M.L.; Klutchko, S. (Pfizer Inc.); Substituted acyl derivatives of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids. DD 201787; EP 0049605; EP 0096157; US 4344949


.........................................................................
2
BENAZEPRIL SYNTHESIS

BENAZEPRIL
CAS NO AS HCl SALT
| 86541-75-5 |
Benazepril, brand name Lotensin (Novartis), is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor.
Benazepril, brand name Lotensin, is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor.
The reaction of 2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (I) with PCl5 in hot xylene gives 3,3-dichloro-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (II), which is treated with sodium acetate and reduced with H2 over Pd/C in acetic acid yielding 3-chloro-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (III). The reaction of (III) with sodium azide in DMSO affords 3-azido-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (IV), which is condensed with benzyl bromoacetate (V) by means of NaH in DMF giving 3-azido-1-(benzyloxycarbonylmethyl)-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (VI). The treatment of (VI) with Raney-Ni in ethanol-water yields 3-amino-1-(benzyloxycarbonylmethyl)-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (VII), which is debenzylated by hydrogenation with H2 over Pd/C in ethanol affording 3-amino-1-(carboxymethyl)-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (VIII). Finally, this compound is condensed with ethyl 3-benzylpyruvate (IX) by means of sodium cyanoborohydride in methanol acetic acid.

REFERENCE
Casta馿r, J.; Serradell, M.N.; CGS-14824 A. Drugs Fut 1984, 9, 5, 317
Ciba-Geigy Corp. (USA). References 1. Watthey, J.W.H. (Ciba-Geigy AG); EP 72.352, GB 2.103.614, JP 83.38.260.

The reaction of 3-bromo-1-phenylpropane (I) with KCN gives 4-phenylbutyronitrile (II), which is hydrolyzed to the corresponding butyric acid (III). The cyclization of (III) with polyphosphoric acid affords 1-tetralone (IV), which is brominated to 2-bromo-1-tetralone (V) and treated with hydroxylamine to give the oxime (VI). The Beckman rearrangement of (VI) yields 3-bromo-2,3,4,5-tetrahydro-1H-(1)benzazepin-2-one (VII), which is treated with sodium azide to afford the azide derivative (VIII). The N-alkylation of (VIII) with ethyl bromoacetate (IX) by means of KOH and tetrabutylammonium bromide in THF gives the N-alkylated azide (X), which is reduced by catalytic hydrogenation to the corresponding amine (XI). The hydrolysis of the ester group of (XI) with NaOH yields the free acetic acid derivative (XII), which is finally reductocondensed with ethyl 2-oxo-4-phenylbutyrate (XIII) by means of sodium cyanoborohydride

WO 2003092698 A1





No comments:
Post a Comment