AZD 9291 Third-generation, oral, irreversible, selective epidermal growth factor receptor (EGFR) inhibitor for Non-small cell lung cancer (NSCLC)

AZD 9291
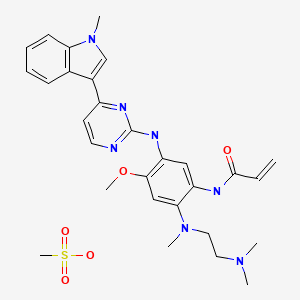
2-Propenamide, N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-
N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylamide.
cas :1421373-65-0, 1421373-66-1(mesylate salt)
Phase II Clinical Trials
cas :1421373-65-0, 1421373-66-1(mesylate salt)
Phase II Clinical Trials
ASTRAZENECA

Mechanism of Action: Third-generation, oral, irreversible, selective epidermal growth factor receptor (EGFR) inhibitor
Non-small cell lung cancer (NSCLC)
AZD-9291 M. Wt: 499.61
AZD-9291 Formula: C28H33N7O2
AZD-9291 Formula: C28H33N7O2
AZD9291, a third-generation orally irreversible epidermal growth factor receptor (EGFR) inhibitor, is under development by British drug maker AstraZeneca for the treatment of patients with metastatic EGFR T790M mutation-positive non-small cell lung cancer (NSCLC).
Lung cancer is the major cause of cancer death in the world while non small cell lung cancer (NSCLC) accounts approx. 85% of all lung cancer diagnosis. Approximately 50% of non–small cell lung cancer (NSCLC) patients who develop resistance to inhibitors of the epidermal growth factor receptor (EGFR) have acquired a second mutation, T790M. There are currently no approved treatments for patients who develop a T790 mutation.
Lung cancer is the major cause of cancer death in the world while non small cell lung cancer (NSCLC) accounts approx. 85% of all lung cancer diagnosis. Approximately 50% of non–small cell lung cancer (NSCLC) patients who develop resistance to inhibitors of the epidermal growth factor receptor (EGFR) have acquired a second mutation, T790M. There are currently no approved treatments for patients who develop a T790 mutation.

AZD9291 mesylate
Also known…
No comments:
Post a Comment