Atipamezole
 4-(2-Ethyl-1,3-dihydroinden-2-yl)-3H-imidazole, Atipamezole, cas 104054-27-5
hydrochloride cas no 104075-48-1
4-(2-Ethyl-1,3-dihydroinden-2-yl)-3H-imidazole, Atipamezole, cas 104054-27-5
hydrochloride cas no 104075-48-1
- MPV 1248 (IS: FarmosGroupLt)
- UNII-03N9U5JAF6 (IS)
- UNII-2W4279571X (IS)
- Pfizer Animal Health ANTISEDAN Product Overview
- Pertovaara A, Haapalinna A, Sirviö J, Virtanen R (2005). "Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist". CNS Drug Reviews 11 (3): 273–88.doi:10.1111/j.1527-3458.2005.tb00047.x. PMID 16389294.
- ANTISEDAN product information page (Pfizer Animal Health)
- Drugs Future, vol. 15, no. 5, 1990, "Atipamezole", pages 448-452, see page 449
Synonyms
1H-imidazole, 4-(2-ethyl-2,3-dihydro-1H-inden-2-yl)- 1H-imidazole, 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)- 5-(2-Ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole Atipamezole 4-(2-Ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole 4-(2-Ethyl-2-indanyl)imidazole 4-(2-Ethyl-indan-2-yl)-1H-imidazole(Atipamezole) 4-(2-ethylindan-2-yl)imidazole Antisedan Antisedan Atipamezol Atipamezolum Atipamezole Hydrochloride CAS 104075-48-1 .................................... Atipamezole is a selective alpha2 - adrenoceptor antagonist which is currently marketed under the trademark Antisedan® for the reversal of sedative- analgesic veterinary drugs. Atipamezole has been disclosed e.g. in the European Patent EP 183492 as useful for the reversal of detomidine. European Patent EP 0589957 discloses the use of atipamezole for the treatment of male sexual impotence. In US 4698692 the use of atipamezole for the attenuation of ethyl alcohol intoxication is disclosed. US Patent No. US6543389 discloses insecticidal pet collars for dogs comprising amitraz and atipamezole. Atipamezole in the collar provides amelioration of amitraz toxicosis in combination with the amitraz in case the dogs ingests the collar. The pet collar comprises 0.01 to 1%, preferably 0.1 to 1 %, by weight of atipamezole. Safe, effective ways to eliminate ectoparasites are desired for the companion animal's well-being, for the well-being and comfort of its human associate and for the prevention of losses in livestock A substantial amount of work has been devoted to identifying the neurotransmitters involved in the facilitation and inhibition of male sexual behaviour (see e.g. Bitran and Hull 1987, Neuroscience and Behavioral reviews 11 , 365-389). Noradrenergic neuro-transmission seems to have an important role. Atipamezole is a selective and potent a2*-adrenoceptor antagonist which is currently marketed for the reversal of sedative-analgesic veterinary drugs. Atipamezole has been disclosed e.g. in the European Patent EP 183492 as useful for the reversal of detomidine. We have now found that this compound is also very effective in increasing male sexual capacity in a monkey model. These findings suggest that atipamezole would be an effective therapy in male impotence in humans as well. Another a2-adrenoreceptor antagonist, yohimbine, is currently used for the treatment of male impotence. Yohimbine increases noradrenergic neurotransmission and has been reported to facilitate the sexual capacity of male animals, although the results of different studies are conflicting. Atipamezole is, however clearly advantageous over yohimbine for this use because of its excellent selectivity. The a2/a-|selectivity ratio of atipamezole is 200-300 times higher than that of yohimbine.- EP 0310745 B (FARMOS OY) 1989.04.12. disclosed preparation of 5-(2-ethyl-2,3-dihydro-1 H-inden-2-yl)-1 H-imidazole salt by two synthetic routes.
-
First synthetic route as starting material was used 2-acetyl-1-indanone, which was alkylated with ethylbromide in acetone in the presence of sodium carbonate to 2-acetyl-2-ethyl-1-indanone. The acetyl group was brominated with bromine in methanol and to imidazole by heating in formamide. Then the intermediate was hydrogenated in 2N hydrochloric acid in the presence of 10% palladium on carbon.
-
Second synthetic route disclosed in the same patent is following, as starting material was used 2,3-dihydro-1H-indene-2-carboxylic acid methyl ester, which was prepared by methylation of 2,3-dihydro-1H-indene-2-carboxylic acid in the presence of sulphuric acid. The 2,3-dihydro-1H-indene-2-carboxylic acid methyl ester was reacted with N-isopropylcyclohexylamide and ethylbromide yielding 2,3-dihydro-2-ethyl-1H-indene-2-carboxylic acid, then thionyl chloride was added and 2,3-dihydro-2-ethyl-1H-indene-2-carboxylic acid chloride was obtained. In the next step ethoxymagnesiummalonic acid ethyl ester in dry ether was added to 2,3-dihydro-2-ethyl-1H-indene-2-carboxylic acid chloride and reaction mixture was treated with sulphuric acid, and 1-(2,3-dihydro-2-ethyl-1H-inden-2-yl)ethanone was obtained, then the intermediate was stirred in methylene chloride and bromine was added by giving a new intermediate 2-bromo-1-(2,3-dihydro-2-methyl-1H-inden-2-yl)ethanone, to which was thereafter added formamide and hydrochloric acid yielding crude product of 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole. The last step involved hydrogenation of the crude product of 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1 H-imidazole with 10% palladium on carbon.
-
EP 0247764 B (ORION-YHTYMÄ OY) 1987.02.12. disclosed the following process for preparation of 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole hydrochloride. The process starts by reaction of alpha, alpha-dibromo-o-xylene with 4-penten-2-one to obtain 1-(2,3-dihydro-2-vinyl-1H-inden-2-yl)ethanone. The obtained intermediate was brominated, e.g. with bromine, methylene chloride was used as solvent and 2-bromo-1-(2,3-dihydro-2-vinyl-1H-inden-2-yl)-ethanone was obtained, which is thereafter reacted with formamide in excess formamide to give a 4(5)-(2,3-dihydro-2-vinyl-1H-inden-2-ylimidazole hydrochloride. As the last step the vinyl group was catalytically hydrogenated to an ethyl group so as to form a product 4(5)-(2,3-dihydro-2-ethyl-1 H-inden-2-yl) imidazole.
-
Another synthetic route for obtaining 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole is disclosed in WAI, Wonf, et al. A Concise Synthesis of Atipamezole. Synthesis. 1995, no.2, p.139-140. The cyclization of alpha, alpha'-dibromo-o-xylene with acetylacetone by means of NaOH and tetrabutylammonium bromide in toluene/water at 80°C under phase-transfer conditions gives the unstable diacetyl derivative, which presumably undergoes cleavage to afford 2-acetylindane. The alkylation of 2-acetylindane with ethyl iodide and potassium tert-butoxide yields 2-acetyl-2-ethylindan, which is brominated with Br2 to give 2-bromoacetyl-2-ethylindan. Finally, this compound is cyclised with formamide at 160°C (some 2-ethyl-2-(4-oxazolyl)indane is also formed but easily eliminated); the cyclization can also be carried out with formamidine in liquid ammonia. Although the substitution of formamide by formamidine acetate eliminates the oxazole formation, it does not increase the yield of Atipamezole (<30%) WAI, Wonf, et al. A Concise Synthesis of Atipamezole. Synthesis. 1995, no.2, p.139-140 in the final step.The preparation of atipamezole hydrochloride salt is described in U.S. Patent 4,689,339, wherein atipamezole obtained from the hydrogenation step is first recovered from alkaline solution as free base. After the evaporation of methylene chloride solvent to dryness the isolated crystalline product is converted into its hydrochloride salt by treatment with dry hydrogen chloride in ethyl acetate

-
- 1. an essential process for obtaining 5-(2-ethyl-2,3-1H-inden-2-yl)-1H-imidazole, without bromination in any step of process, thus preventing the possibility of brominated by-products;
- 2. This process has given superior yields, compared to patents cited above;
- 3. This process is amenable to large scale production which does not require specialized equipment.
-
The condensing of commercially available 1-trityl-1H-imidazole-4-carboxaldehyde (I) with phtalide to form 2-(1-trityl-1H-imidazole-4-yl)indan-1,3-dione (II) is performed under the conditions that are similar to those used for synthesis of 4-(indane-1,3-dionyl) pyridine J. Org. Chem. 1971, vol.36, p.1563. surprisingly, the bulky 1-trityl-1H-imidazole-4-carboxaldehyde (I) reacted as expected and produced 2-(1-trityl-1H-imidazole-4-yl)indan-1,3-dione (II) in over 67% yield. Both ethyl acetate and dioxane can be used as reaction media.
-
The alkylation of (II) by ethyl iodide is performed in boiling acetone with potassium carbonate as basic agent. 2-Ethyl-2-(1-trityl-1H-imidazole-4-yl)indan-1,3-dione (III) is formed in over 67% yield and easily isolated from the acetone solution by concentrating it and diluting with water. A high purity (III) is obtained after crystallization from methanol or ethanol.
-
Removing the trityl group of 2-ethyl-2-(1-trityl-1H-imidazole-4-yl)indan-1,3-dione by acid hydrolysis to yield the deprotected 2-ethyl-2-(1H-imidazol-2-yl)indan-1,3-dione.
-
The reduction of (IV) to 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole hydrochloride (V) is performed in hydrogenation apparatus with Pd/C catalyst under hydrogen pressure in HCI solution. The reaction proceeds under variable pressure and temperature conditions, but a pressure of about 3 bar and the temperature of about 80-85°C is preferable. After removing the catalyst the product crystallizes on chilling in over 77% yield. It can be purified by additional crystallization.
-
EP 0310745 B (FARMOS OY) 1989.04.12. disclosed preparation of 5-(2-ethyl-2,3-dihydro-1 H-inden-2-yl)-1 H-imidazole salt by two synthetic routes.
-
First synthetic route as starting material was used 2-acetyl-1-indanone, which was alkylated with ethylbromide in acetone in the presence of sodium carbonate to 2-acetyl-2-ethyl-1-indanone. The acetyl group was brominated with bromine in methanol and to imidazole by heating in formamide. Then the intermediate was hydrogenated in 2N hydrochloric acid in the presence of 10% palladium on carbon.
-
Second synthetic route disclosed in the same patent is following, as starting material was used 2,3-dihydro-1H-indene-2-carboxylic acid methyl ester, which was prepared by methylation of 2,3-dihydro-1H-indene-2-carboxylic acid in the presence of sulphuric acid. The 2,3-dihydro-1H-indene-2-carboxylic acid methyl ester was reacted with N-isopropylcyclohexylamide and ethylbromide yielding 2,3-dihydro-2-ethyl-1H-indene-2-carboxylic acid, then thionyl chloride was added and 2,3-dihydro-2-ethyl-1H-indene-2-carboxylic acid chloride was obtained. In the next step ethoxymagnesiummalonic acid ethyl ester in dry ether was added to 2,3-dihydro-2-ethyl-1H-indene-2-carboxylic acid chloride and reaction mixture was treated with sulphuric acid, and 1-(2,3-dihydro-2-ethyl-1H-inden-2-yl)ethanone was obtained, then the intermediate was stirred in methylene chloride and bromine was added by giving a new intermediate 2-bromo-1-(2,3-dihydro-2-methyl-1H-inden-2-yl)ethanone, to which was thereafter added formamide and hydrochloric acid yielding crude product of 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole. The last step involved hydrogenation of the crude product of 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1 H-imidazole with 10% palladium on carbon.
-
EP 0247764 B (ORION-YHTYMÄ OY) 1987.02.12. disclosed the following process for preparation of 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole hydrochloride. The process starts by reaction of alpha, alpha-dibromo-o-xylene with 4-penten-2-one to obtain 1-(2,3-dihydro-2-vinyl-1H-inden-2-yl)ethanone. The obtained intermediate was brominated, e.g. with bromine, methylene chloride was used as solvent and 2-bromo-1-(2,3-dihydro-2-vinyl-1H-inden-2-yl)-ethanone was obtained, which is thereafter reacted with formamide in excess formamide to give a 4(5)-(2,3-dihydro-2-vinyl-1H-inden-2-ylimidazole hydrochloride. As the last step the vinyl group was catalytically hydrogenated to an ethyl group so as to form a product 4(5)-(2,3-dihydro-2-ethyl-1 H-inden-2-yl) imidazole.
-
Another synthetic route for obtaining 5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole is disclosed in WAI, Wonf, et al. A Concise Synthesis of Atipamezole. Synthesis. 1995, no.2, p.139-140. The cyclization of alpha, alpha'-dibromo-o-xylene with acetylacetone by means of NaOH and tetrabutylammonium bromide in toluene/water at 80°C under phase-transfer conditions gives the unstable diacetyl derivative, which presumably undergoes cleavage to afford 2-acetylindane. The alkylation of 2-acetylindane with ethyl iodide and potassium tert-butoxide yields 2-acetyl-2-ethylindan, which is brominated with Br2 to give 2-bromoacetyl-2-ethylindan. Finally, this compound is cyclised with formamide at 160°C (some 2-ethyl-2-(4-oxazolyl)indane is also formed but easily eliminated); the cyclization can also be carried out with formamidine in liquid ammonia. Although the substitution of formamide by formamidine acetate eliminates the oxazole formation, it does not increase the yield of Atipamezole (<30%) WAI, Wonf, et al. A Concise Synthesis of Atipamezole. Synthesis. 1995, no.2, p.139-140 in the final step.
Atipamezole [5-(2-ethyl-2,3-dihydro-1H-inden-2-yl)-1H-imidazole, 1] is a veterinary drug that has been investigated for treating Parkinson's disease in humans. V. Lusis and co-inventors summarize several ways to synthesize 1. Some routes give a low yield of 1 and produce large quantities of an oxazole byproduct. Other processes involve a sluggish bromination reaction that leads to many byproducts.
The inventors’ process is intended to overcome these problems. In particular, it does not use the bromination reaction and thus avoids forming brominated byproducts. The process, outlined in the figure, begins with the reaction of imidazole 2 with i-PrMgCl to form iodo Grignard reagent 3, which is treated with DMF to give 4. This intermediate is not isolated but is treated with aq NH4Cl to give aldehyde 5, isolated in 73.2% yield. The aldehyde is condensed with phthalide (6) in the presence of NaOMe to produce imidazolylindane 7, recovered in crude form in 67.2% yield.

In the next stage, compound 7 is alkylated with EtI in the presence of K2CO3. Product 8 is isolated in 50.9% yield after being recrystallized from EtOH. Product1 can be produced directly from 8 by making its HCl salt and hydrogenating the salt over Pd/C. Crude atipamezole is isolated as its HCl salt in 26.6% yield.
Alternatively, acid hydrolysis of 8 removes the trityl group to form dione 9, recovered as a white crystalline solid in 76.2% yield. The HCl salt of 9 is then hydrogenated to 1·HCl.
The patent’s claims cover the process to make 1 and new compounds 7 and 8. The overall yield of compound 1 is poor, partly because of the low yield from the hydrogenation step. The inventors claim, however, that the yield is higher than from earlier methods. They point out that the process is amenable to large-scale production without the use of specialized equipment. (JSC Grindeks [Riga, Latvia]. US Patent 8,431,717, April 30, 2013; Keith Turner), View the full-text here.
....................................
nmr
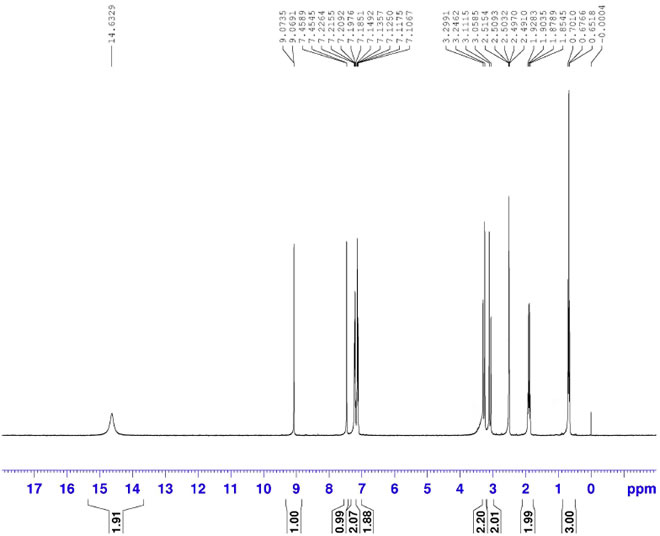 Atipamezole Hydrochloride CAS 104075-48-1 HNMR
................................................................
Atipamezole Hydrochloride CAS 104075-48-1 HNMR
................................................................

 Atipamezole Hydrochloride CAS 104075-48-1 HNMR
................................................................
Atipamezole Hydrochloride CAS 104075-48-1 HNMR
................................................................


No comments:
Post a Comment